Mihir S. Wagh
DEFINITION
■ Endoscopic ultrasonography (EUS or echoendoscopy or endosonography) is an endoscopic procedure using a high-frequency ultrasound transducer integrated into an endoscope.
■ The echoendoscope is an oblique-viewing endoscope. It has an ultrasound transducer located at its tip, which emits high-frequency acoustic waves to the surrounding tissues. This action permits the acquisition of unique views of the gut wall, the surrounding organs, and blood vessels.
BACKGROUND
■ EUS was first introduced by Dr. Eugene DiMagno in the 1980s. Initial echoendoscopes were radial, which scan perpendicular to the axis of the endoscope and provide 360-degree images similar to computed tomography (CT). In 1991, a convex linear-array echoendoscope was introduced. These linear echoendoscopes scan parallel to the longitudinal axis of the endoscope and enable fine needle aspiration (FNA) and various therapeutic interventions.
■ Over the past two decades, EUS has evolved to become an indispensible tool for the evaluation of the pancreas. EUS is useful for the detection, diagnosis, staging, and treatment of multiple pancreatic disorders including solid pancreatic masses, pancreatic cystic lesions, and acute and chronic pancreatitis.
■ Currently, two types of echoendoscopes are available: radial sector and linear-array transducers. Radial and linear EUS provides high-resolution images of the gut lumen, the adjacent organs, the lymph nodes, and the vascular structures. They are also equipped with color Doppler, which allows for accurate identification of vascular structures and aids in vascular staging of pancreatic tumors.
■ EUS is a safe procedure. Complications are rare and typically related to therapeutic efforts. EUS-FNA complications include bleeding (0% to 1.3%), perforation (0% to 0.4%), infection (0.3%), and pancreatitis (1% to 2%).1,2 The risk of bacteremia is low, and prophylactic antibiotics are not recommended except prior to EUS-guided FNA of pancreatic cystic lesions. The risk of tumor seeding is significantly lower as compared to percutaneous approaches with only four case reports so far.2
PANCREATIC DISORDERS AND ENDOSCOPIC ULTRASONOGRAPHY FINDINGS
■ Pancreatic tumors typically appear as irregularly shaped hypoechoic or heterogeneous areas within the normal echo texture of the pancreas. In cases of pancreatic cancer, tumor extension into the adjacent vasculature (portal; splenic and superior mesenteric veins; and celiac, splenic, and superior mesenteric arteries), the common bile duct, and the duodenum can be identified. Additionally, ascites, celiac and peripancreatic lymph nodes, and metastatic disease to the liver can be seen.
■ Pancreatic cysts are divided into nonneoplastic and neoplastic cysts. EUS findings should be interpreted in the context of the clinical impression, morphologic EUS criteria, and results of the cystic fluid analysis to determine the origin of the cyst and guidance of further management. Morphologic features associated with an increased risk for malignancy are an irregular or thickened cyst wall, the presence of mural nodules or a solid mass within the cyst, a cyst size over 3 cm, or an increase in cyst size during follow-up.
■ EUS can help identify causes of pancreatitis, including gallbladder and bile duct stones and sludge (microlithiasis), pancreatic duct stones, pancreatic cysts (pseudocysts and cystic neoplasms), ampullary neoplasms, pancreas divisum, changes of chronic pancreatitis, and pancreatic masses.
■ EUS is sensitive to subtle changes associated with chronic pancreatitis. It shows fine parenchymal details such as early fibrosis, calcifications, and pancreatic ductal changes. The diagnosis of chronic pancreatitis by EUS depends on the presence or absence of multiple EUS criteria of chronic pancreatitis (Rosemont classification),3 which includes parenchymal features of echogenic foci, focal regions of increased echogenicity (strands), lobularity, cyst formation, and ductal EUS features (increased echogenicity of the main pancreatic duct wall, main pancreatic duct calculi, irregular contour of the main pancreatic duct, dilation of the main pancreatic duct, and side branch dilation).
OTHER DIAGNOSTIC STUDIES
■ In the 1980s, EUS was developed to overcome the limitations of transabdominal ultrasonography (US) and CT scan imaging of the pancreas.
■ EUS provides high-resolution images of the pancreatic duct, as well as the parenchyma, and complements the ductal images seen in endoscopic retrograde cholangiopancreatography (ERCP).
■ EUS is considered one of the most sensitive imaging modalities to detect pancreatic tumors and has the additional advantage of acquiring tissue samples by FNA with linear endosonography. The sensitivity of EUS ranges from 93% to 100%, whereas for CT, it ranges from 53% to 92%.4
■ Compared to US- or CT-guided FNA, EUS-FNA has been proven to be the safest technique with comparable accuracy (76% of EUS vs. 81% of US/CT).5 The sensitivity of EUS-FNA for diagnosing pancreatic cancer is greater than 90% with a specificity of greater than 95%.4
MANAGEMENT
Preoperative Planning
■ A recent complete blood count, basic electrolytes, and coagulation studies are indicated prior to EUS. The patient should hold anticoagulants and antiplatelet agents or use bridge therapy with heparin before EUS-guided FNA.
■ Patients are normally instructed to fast after midnight, the night before the procedure.
■ Prophylactic antibiotics are recommended only for EUS-guided FNA of cystic lesions.6
■ The procedure is generally performed under conscious sedation or monitored anesthesia care based on presence of associated medical comorbidities.
Positioning
■ EUS of the pancreas is usually performed with the patient in the left lateral decubitus position, similar to that for performing an esophagogastroduodenoscopy (EGD).
■ If drainage of pancreatic fluid collections is planned, a prone position is preferred to facilitate better fluoroscopic visualization without interference from bony structures; this positioning also fosters the concomitant performance of ERCP if indicated.
TECHNIQUES
ESOPHAGEAL INTUBATION
■ The echoendoscope has an oblique endoscopic view, and therefore, pharyngeal intubation is typically blind or with partial views. The echoendoscope is inserted into the pharynx with downward deflection of the tip, which is then straightened using the up-down wheel once the pharynx is reached. The esophagus is intubated with a slight clockwise torque. Care needs to be taken so as not to forcefully intubate the esophagus because of the risk of perforation if any anatomic variant is present.
STOMACH STATION
■ This is the station for examination of the body and tail of the pancreas.
■ The echoendoscope is positioned distal to the gastroesophageal (GE) junction, and the aorta is located.
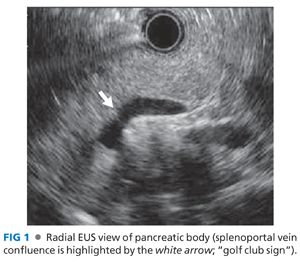
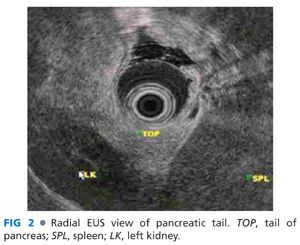
■ Radial echoendoscopy: The aorta is seen as a circular anechoic structure with a positive Doppler signal. The balloon is inflated and keeping the aorta at the 6 o’clock position, and the echoendoscope is passively advanced. The diaphragmatic crura are seen as hypoechoic structures that wrap around the aorta. With further advancement, the crura disappear and the celiac trunk emerges from the aorta at 12 o’clock position and soon bifurcates into the hepatic and splenic arteries, which looks like a “whale’s tail.” Advancement of the echoendoscope from this position leads to the pancreas body with its characteristic salt-and-pepper appearance. Deep to the pancreas is the classic anechoic Doppler-positive club head (golf club sign), which signifies the splenoportal confluence (FIG 1). Clockwise torque and gradual withdrawal of the scope is performed to image the body up to the tail of the pancreas. Initially, the left kidney is seen below the pancreas as an oval structure with a hypoechoic cortex and a hyperechoic medulla. Next, the spleen is seen as a hypoechoic structure to the right of the screen, and the splenic artery and vein are seen entering the hilum of the spleen. This completes the exam up to the tail of the pancreas (FIG 2). From this region, the maneuvers are reversed and counterclockwise torque and gradual advancement of the scope is performed to image the pancreas up to the neck.
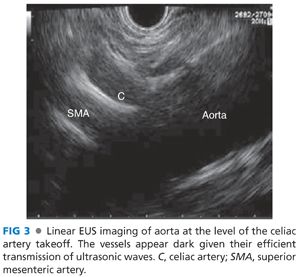
■ Linear echoendoscopy: The approach is similar to that described earlier with the radial echoendoscope. The echoendoscope is positioned at the GE junction and the scope is rotated in a clockwise manner to locate the aorta, which is a linear structure that slopes downward from the right to the left of the screen. The diaphragmatic crura are again noted and the celiac artery is seen arising from the aorta as soon as the crura disappear (FIG 3). The celiac artery is traced until it splits into the hepatic and splenic artery. The superior mesenteric artery is seen emerging from the aorta distal to the takeoff of the celiac artery. Advancement of the scope beyond the celiac bifurcation with a gentle down on the up-down wheel (tip up of the echoendoscope) leads to the pancreas. The pancreas is then traced to the tail and the splenic hilum by gentle clockwise rotation and withdrawal. The maneuvers are then reversed to trace the pancreas up to the neck. Unlike the radial endoscope where advancement and withdrawal is mostly passive, constant slow clockwise and counterclockwise torque is essential when examining with a linear endosonoscope.
DUODENUM STATION (BULB, AMPULLA, AND DISTAL DUODENUM)
■ The head of the pancreas is examined from the bulb, the ampullary region, and from the duodenum distal to the ampulla (FIG 4).
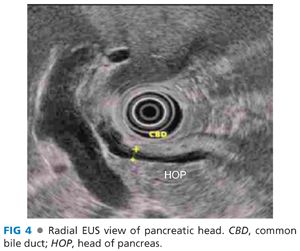
■ Radial echoendoscopy: The scope is gently advanced through the stomach and the pylorus is visualized endoscopically. The scope is then advanced through the pylorus with the tip up, that is, big wheel pushed toward the endoscopist (“setting sun sign”). The balloon on the echoendoscope is inflated in the duodenal bulb and endosonographic visualization is commenced. The liver is positioned at 11 o’clock, which places the pancreas at 6 o’clock position. The portal vein is seen as a large tubular structure coursing downward from the top left to the bottom right of the screen. The duodenal falloff represents the muscularis propria of the duodenum and is a hypoechoic line coursing down from the transducer. The bile duct is a tubular anechoic structure coursing from the liver to the duodenal falloff and is located between the portal vein and the transducer. The scope is pushed in with a clockwise torque to trace the bile duct to the ampulla. Counterclockwise torque and withdrawal of the scope enables visualization of the bile duct up to the hilum (bifurcation). The pancreatic duct may or may not be seen in the same plane as the portal vein, bile duct, and the duodenal falloff and gentle upward or downward deflection may be needed to achieve adequate imaging of the pancreatic duct. The next position for visualization of the head of the pancreas is at the level of the ampulla. The echoendoscope is passed into the second part of the duodenum and then is withdrawn into a short position akin to when performing an ERCP. The major papilla is visualized and the balloon is inflated. The major papilla is positioned such that upward deflection of the scope should bring the scope near the papilla. The pancreas appears crescent-shaped in this position. As the transducer is moved back and forth, the bile and pancreatic duct appear to course through the duodenal wall with the bile duct appearing in a cross-sectional configuration above the pancreatic duct. Duodenal motility can be reduced with intravenous administration of glucagon and water infused into the duodenal lumen, if detailed views of the ampulla are required. The uncinate process is next visualized by advancing the scope just distal to the papilla (FIG 5
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








