David L. Bartlett
DEFINITION
■ The use of robotic-assisted surgery for liver resections is mostly dependent on tumor location and the experience of the surgical team. Indications for the use of robotics have been reported in the Louisville Statement in 2008, which set forth the guidelines for laparoscopic liver resection. Patients with solitary lesions, 5 cm or less, and located in segments 2 to 6 were considered eligible for robotic-assisted surgery. The use of robotics should not compromise the aforementioned principles or nomenclatures.1
■ The indication has been extended by most experienced robotic liver surgeons to include all resections, which would be considered for laparoscopic resection. The contraindications include large lesions that will prevent safe mobilization of the liver without an open incision. The space in an insufflated abdomen may not be enough to allow for manipulation of an exceedingly large tumor. The tumors that are close to major intrahepatic pedicles may also be unsafe to approach minimally invasively. Also, the sick livers with high intrahepatic portal venous pressure will lead to excessive blood loss and may not be appropriate for a minimally invasive approach.
DIFFERENTIAL DIAGNOSIS
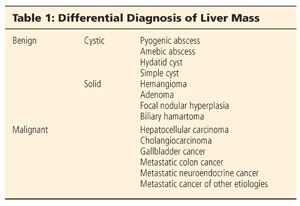
■ The differential diagnosis of a liver mass includes both benign and malignant processes. Benign processes are either cystic or solid. Malignant processes are usually solid (Table 1).
PATIENT HISTORY AND PHYSICAL FINDINGS
■ The evaluation of a patient with a liver mass should include a complete history and physical exam.
■ History should include questions regarding presence of abdominal pain, weight loss, alcohol use, viral hepatitis, liver disease, tattoos, blood transfusions, personal history of cancer, family history of liver, and colon cancer. History should include use of oral contraceptives for women.
■ Physical exam should be directed toward findings such as presence of abdominal mass, hepatomegaly, splenomegaly, ascites, jaundice, scleral icterus, flapping tremor, and caput medusa.
LABORATORY TESTS AND IMAGING
■ Workup should include blood sent for the following:
■ Complete blood count
■ Platelet count
■ Blood urea nitrogen
■ Creatinine
■ Electrolytes
■ Liver enzymes
■ Albumin
■ International normalized ratio (INR)
■ Viral hepatitis screen
■ Serum ammonia level
■ Tumor markers (carcinoembryonic antigen, α-fetoprotein, cancer antigen 19-9)
■ Imaging studies play a great role in the diagnoses of liver lesions and in the preoperative planning for liver resection.
■ Ultrasound differentiates between solid and cystic lesions of the liver. It is usually used as a screening modality in patients with right upper quadrant pain. Its use is limited by interference secondary to presence of bowel gas, obesity, and overlying ribs.
■ Contrast-enhanced, multiphasic computed tomography (CT) scans are used for diagnosis of liver lesions and preoperative planning for liver resections. These include arterial, venous, and portal phases. Their value lies in their ability to detect proximity of lesions to major arterial and portal structures and to asses for resectability.
■ Volumetric analysis of functional liver remnant is used to assess feasibility of resection, and data supports its use because the remaining liver volume has been shown to predict mortality in cirrhotic patients. In general, a functional liver remnant of 20% is desired with a healthy liver and 50% with a diseased liver.
■ Contrast-enhanced magnetic resonance imaging (MRI) is another imaging modality that is gaining acceptance for evaluation and characterization of liver lesions. It allows for better contrast resolution, avoidance of radiation exposure, and multiplanar imaging. The choice of CT versus MRI is institution dependent.
■ Other imaging modalities used include positron emission tomography (PET) scan and CT-PET scans. PET imaging is ideal for many metastatic cancers to the liver, but it is best combined with an intravenous (IV) contrast CT scan so that anatomic relations can be made.
SURGICAL TECHNIQUE
■ Patient positioning and room setup:
■ The robot is usually at the top of the operating table. The anesthesia area is on the left side of the patient’s head.
■ The patient is placed in the supine position with the legs split and both arms tucked.
■ The first surgeon stands on the right side of the table while the second surgeon stands between the legs. An assistant stands on the left side of the table.
■ The patient is placed at 30-degrees reverse Trendelenburg at all times during the procedure (FIG 1).

TECHNIQUES
RIGHT HEPATECTOMY
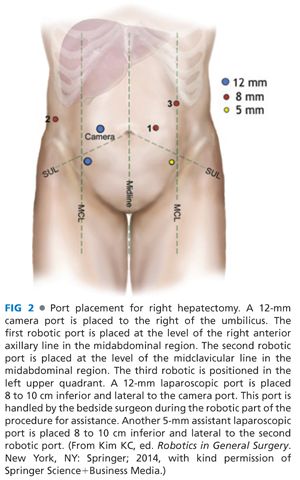
■ Port placement
■ Pneumoperitoneum is established through placement of a 5-mm port in the left upper quadrant. This will be exchanged at the end of port placement to become the third robotic port.
■ A 12-mm camera port is placed to the right of the umbilicus.
■ First robotic port is placed at the level of the right anterior axillary line in the midabdominal region.
■ Second robotic port is placed at the level of the midclavicular line in the midabdominal region.
■ A 12-mm laparoscopic port is placed 8 to 10 cm inferior and lateral to the camera port. This port is handled by the bedside surgeon during the robotic part of the procedure for assistance.
■ Another 5-mm assistant laparoscopic port is placed 8 to 10 cm inferior and lateral to the second robotic port (FIG 2).
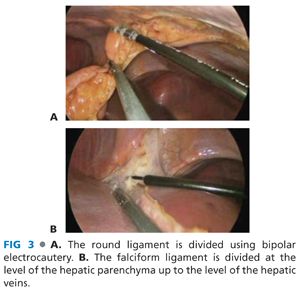
■ Step 1 (laparoscopic): The falciform and round ligaments are divided using electrocautery. This exposes the anterior surface of the liver up to the fibroareolar tissue investing the hepatic veins (FIG 3).
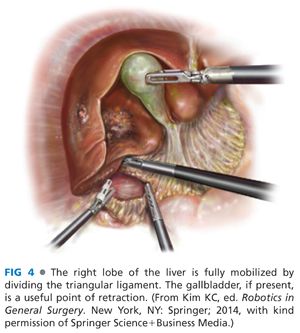
■ Step 2 (laparoscopic): The patient is positioned right side up by rotating the operating table. The hepatic flexure is mobilized to inferiorly reflect the colon. The right lobe of the liver is retracted superiorly. The duodenum is reflected medially. Gerota’s fascia is retracted inferiorly and the right triangular and coronary ligaments are divided up to the right hepatic vein (FIG 4).
■ Step 3 (laparoscopic): Intraoperative ultrasound (IOUS) of the liver is then performed using the 12-mm laparoscopic port. This locates the mass in relation to the segmental anatomy of the liver and drives the planning of the resection plane.
■ Step 4 (laparoscopic): The short hepatic/caudate veins that insert into the retrohepatic vena cava are divided between clips or with a coagulation device.
■ Step 5 (laparoscopic): The right hepatic vein is encircled with a vessel loop.
■ Step 6: Docking of the robot is performed using the first, second, and third robotic ports for the respective robotic arms. The camera is aligned with the head at this time.
■ Step 7: The gallbladder, if present, is retracted cephalad and blunt dissection of triangle of Calot performed so that both cystic artery and duct are identified (FIG 5
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








