Chapter 58 Electronically Enhanced Endoscopic Imaging
Introduction
Although ex vivo histology remains the current standard of confirming all lesions, the concept of real-time in vivo histology of lesions or optical biopsies during ongoing endoscopy has been introduced.1 In vivo histology could play an important role in a busy GI endoscopy practice not only in diagnosing small lesions of otherwise unknown significance before making a decision regarding their necessary resection but also in targeting biopsies and directing treatment decisions during surveillance endoscopic evaluation, such as in inflammatory bowel disease or Barrett’s esophagus.
Electronically enhanced endoscopy is also expected to improve lesion detection rates. Conventional white light videoendoscopy was found to be associated with alarming miss rates for subtle lesions (e.g., flat and depressed adenomas).2 Even experienced gastroenterologists can miss 6% of advanced adenomas and 30% of all adenomas.3,4 Some studies confirmed the development of colorectal cancer, especially in the right site of the colon, despite a surveillance colonoscopy program.5,6 Because subtle dysplastic and early neoplastic lesions are often not easily recognized during regular standard white light endoscopy, electronically enhanced endoscopic technologies are intended to improve their detection. These electronically enhanced imaging technologies including broad field and point fields (Table 58.1) combined together are expected to improve diagnostic yield and to allow in vivo histology diagnosis, minimize the burden of unnecessary biopsies, and lead to immediate decisions for definitive management.
Table 58.1 Methods of Enhanced Endoscopic Imaging
| Methods | Technology | Aims |
|---|---|---|
| Broad field electronic enhancement technologies | Virtual chromoendoscopy: NBI, i-Scan, AFI | Increased mucosal contrast and detection of flat and depressed lesions |
| Point field electronic enhancement technologies | Endoscope-based CLE, probe-based CLE | In vivo histology |
AFI, autofluorescence imaging; CLE, confocal laser endomicroscopy; NBI, narrow band imaging.
Broad Field Image Enhancement Techniques: High-Resolution Endoscopy, Virtual Chromoendoscopy, and Other Optical Techniques
Broad image enhanced endoscopy (IEE) technologies include traditional dye-based IEE with chromoendoscopy and optical methods including equipment-based IEE with narrow band imaging (NBI), electronically based IEE with spectral estimation technologies such as Fuji Intelligent Chromo Endoscopy (FICE) and i-Scan, and autofluorescence imaging (AFI).7 These technologies are used with more recently developed high-resolution endoscopes.
High-Resolution and High-Definition Endoscopy
High-definition (HD) systems can display 1080 scanning lines on a screen compared with standard definition analog systems, which can generate 576 scanning lines. These HD endoscopes with high-density couple charge devices, combined with HD 1080-line television monitors, produce images with increased spatial resolution. A high-definition imaging system was used in more recent trials and showed very high adenoma detection rates greater than 50% in screening patients with average colorectal cancer risk.8–11
The use of HD colonoscopy was associated with a higher adenoma detection rate compared with SD colonoscopy in a large cohort study of 2430 patients.12 This higher rate was most apparent for smaller adenomas (<10 mm) and adenomas in the left colon. Other colonoscopic studies comparing standard definition white light endoscopy with HD white light endoscopy have revealed mixed results.11,13–15 In a study by East and colleagues11 of 132 patients undergoing routine screening colonoscopies by one dedicated and highly experienced endoscopist, adenoma detection rate was higher with HD colonoscopy (71% vs. 60%), but this trend did not reach statistical significance in the group of 58 HD patients and 72 standard definition patients. Pellise and coworkers13 showed no statistically significant advantage in detection of adenomas (HD, 26%; standard definition, 25%) and all polyps (HD, 43%; standard definition, 38%) using a HD colonoscope versus a standard definition colonoscope in 639 patients undergoing routine screening colonoscopy. However, this study was underpowered to detect small differences between groups in a population with a prevalence of adenomas of less than 30%, which is typical of average-risk individuals.
Narrow Band Imaging
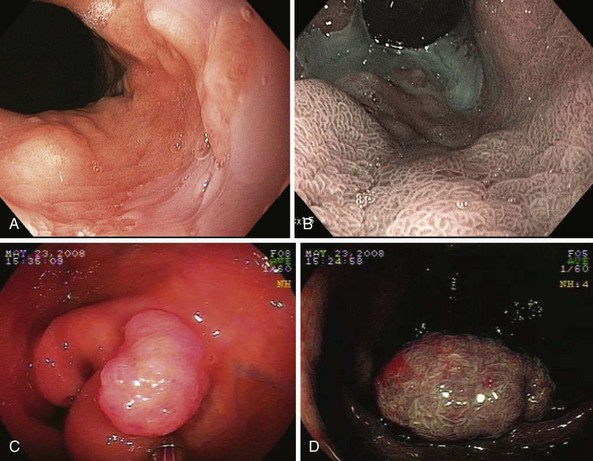
NBI (Olympus Corp, Tokyo, Japan) is a method of virtual chromoendoscopy that has a potential to improve detection of mucosal abnormalities without time-consuming and impractical application of chromic agents. NBI is currently the most investigated advanced endoscopic imaging technique for the detection of Barrett’s dysplasia and colorectal polyps (Fig. 58.1). Conventional white light endoscopy uses the full visible wavelength range (400 to 700 nm) to produce a red/green/blue image, whereas NBI combined with magnification endoscopy illuminates the tissue surface using special filters that narrow the red/green/blue bands and simultaneously increases the relative intensity of the blue band.
The technology was developed by Gono and associates16 in 1999 as a joint project of the Japanese National Cancer Center Hospital East and Olympus Corporation. The investigators studied variations of conventional endoscopy that potentially could visualize early changes of angiogenesis associated with the development of dysplasia and superficial neoplasia. Using light filters, the contribution of blue light is increased by narrowing the band widths of the red, green, and blue components of the excitation light and reducing the amount of green light and eliminating the red light. The resulting “narrow band” blue/green light improves imaging of mucosal patterns because of the limited optical scattering and shallow penetration depth. This blue light is also absorbed by hemoglobin (because the hemoglobin absorption band [Soret band] lies at 415 nm) for optimal detection of mucosal glandular and vascular patterns and the presence of abnormal blood vessels that are associated with the development of dysplasia.
Numerous studies have shown the value of this technology in the evaluation of patients with upper GI lesions including Barrett’s esophagus dysplasia and gastric lesions.17,18 The systematic review by Curvers and associates18 summarized performance and clinical utility of NBI in upper GI endoscopy with a focus on the primary detection of premalignant lesions and the differentiation between neoplastic and nonneoplastic lesions.
A prospective, blinded, tandem study in 65 patients referred for evaluation of Barrett’s dysplasia by Wolfsen and colleagues17 showed that in patients evaluated for Barrett’s esophagus with dysplasia, NBI detected significantly more patients with dysplasia and higher grades of dysplasia with fewer biopsy samples compared with standard resolution endoscopy. Specifically, NBI-targeted biopsies found dysplasia in more patients (37 patients [57%]) compared with standard resolution endoscopy with targeted plus random biopsies (28 patients [43%]; P < .001). NBI also found higher grades of dysplasia in 12 patients (18%) compared with no cases in which standard resolution endoscopy with targeted and random biopsies detected a high grade of histology (0%; P < .001). In addition, more biopsy samples were taken using standard resolution endoscopy with targeted plus random biopsies (mean 8.5 biopsy samples per case) compared with NBI-directed biopsies (mean 4.7 biopsy samples per case; P < .001). This ability of high-resolution endoscopy combined with NBI to detect dysplasia in significantly more patients with Barrett’s esophagus using fewer biopsy specimens supports the role of this technology in surveillance evaluation.
NBI technology was also evaluated for the detection of colorectal lesions.19,20 More recent studies revealed conflicting results with overall no improvement of adenoma detection rates.9,21,22 In a study by Adler and coworkers23 of 401 patients randomly assigned to undergo either standard definition colonoscopy or NBI colonoscopy, a higher adenoma detection rate in the NBI group compared with the standard group (23% vs. 17%) was shown in the initial phase of the study, suggesting a learning effect with the introduction of a new method influencing the conventional technology as well.
The use of NBI for the detection of dysplasia in long-standing ulcerative colitis has not led to improvement of neoplasia detection.24 East and colleagues25 showed that NBI improved adenoma detection in high-risk patients with hereditary nonpolyposis colorectal cancer syndrome, in whom a second additional colonoscopic examination with NBI doubled the total number of adenomas detected. A systematic review by van den Broek and coworkers22 summarized data on the performance and clinical utility of NBI during colonoscopy. Their review did not show a significant improvement in adenoma detection using NBI, but it confirmed the value of NBI for differentiation of neoplastic from nonneoplastic colonic polyps when used by experts.
The main advantage of NBI seems to be not detection but rather characterization because NBI has a high sensitivity of 90% to 95% and a specificity of 80% to 85% for differentiation of neoplastic from nonneoplastic lesions.26 Based on available studies, this level of accuracy is comparable to that of expert chromoendoscopists.27,28 It is anticipated that with appropriate training, NBI will be used routinely for lesion discrimination and management.
More recent trials confirmed very high accuracy of NBI for the diagnosis of small colorectal lesions (<10 mm) in selected cases of high-quality and high-confidence stored images interpreted offline.29,30 Rex29
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree