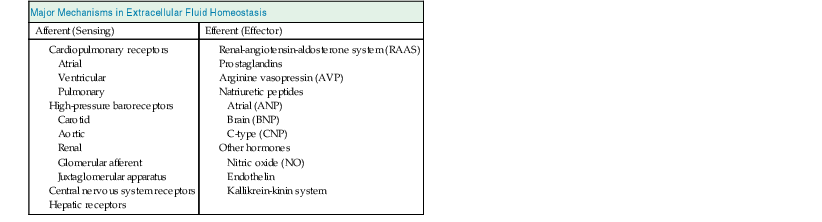
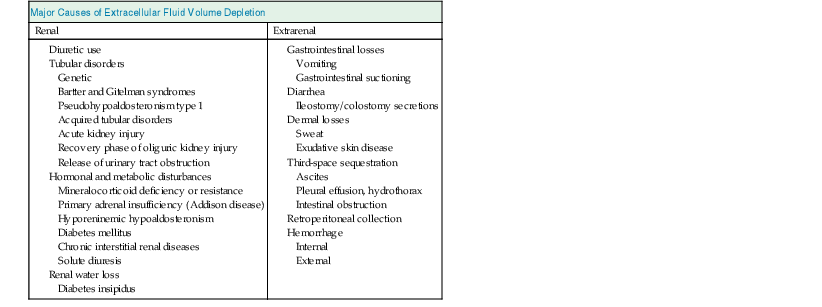
Elwaleed A. Elhassan, Robert W. Schrier Water is the predominant constituent of the human body. In healthy individuals, it makes up 60% of a man’s body weight and 50% of a woman’s body weight. Body water is distributed in two compartments: the intracellular fluid (ICF) compartment, containing 55% to 65% of body water, and the extracellular fluid (ECF) compartment, containing the remaining 35% to 45%. The ECF is further subdivided into two spaces: the interstitial space accounts for about three fourths of ECF, and the intravascular space represents the remaining fourth (Table 7-1). Table 7-1 Composition of body fluid compartments. The table indicates the relative size of the compartments and their approximate absolute volume (in liters) in a 70-kg adult. Electrolyte concentrations are shown in milimoles per liter. Total body water diffuses freely between the intracellular space and the extracellular spaces in response to solute concentration gradients. Therefore the amount of water in different compartments depends entirely on the quantity of solute in that compartment. The major solute in the ECF is sodium ion (Na+), and the major intracellular solute is potassium ion (K+). The maintenance of this distribution is fulfilled by active transport through the Na+,K+–adenosine triphosphate (ATP)–dependent pumps on the cell membrane, and this determines the relative volume of different compartments. Because sodium is the predominant extracellular solute, the ECF is determined primarily by the sodium content of the body and the mechanisms responsible for its maintenance. The amount of sodium is therefore tightly regulated by modulation of renal retention and renal excretion in situations of deficient and excess ECF, respectively. Fluid movement between the intravascular and interstitial spaces of the ECF occurs across the capillary wall and is governed by Starling forces, namely, the capillary hydrostatic pressure and colloid osmotic pressure. The transcapillary hydrostatic pressure gradient exceeds the corresponding oncotic pressure gradient, thereby favoring movement of plasma ultrafiltrate into the extravascular compartment. The return of fluid into the intravascular compartment occurs through lymphatic flow. Maintaining the ECF volume determines the adequacy of the circulation and in turn the adequacy of delivery of oxygen, nutrients, and other substances needed for organ functions as well as removal of waste products. This is achieved despite day-to-day variations in the intake of sodium and water, with the ECF volume varying by only 1% to 2%. The term effective arterial blood volume is used to describe the blood volume detected by the sensitive arterial baroreceptors in the arterial circulation. The effective arterial blood volume (EABV) can change independently of the total ECF volume. EABV can explain the sodium and water retention in different clinical situations (see later discussion). Circulatory stability depends on a meticulous degree of ECF homeostasis. The operative homeostatic mechanisms include an afferent sensing limb, comprising several volume and stretch detectors distributed throughout the vascular bed, and an efferent effector limb. Adjustments in the effector mechanisms occur in response to afferent stimuli by sensing-limb detectors, to modify circulatory parameters. Disorders of either sensing mechanisms or effector mechanisms can lead to failure of adjustment of sodium handling by the kidney, with resultant hypertension or edema formation in the patient with positive sodium balance, or hypotension and hypovolemia in the patient with negative sodium balance. Afferent limb (sensing) sites include low-pressure cardiopulmonary receptors (atrial, ventricular, and pulmonary stretch receptors), high-pressure arterial baroreceptors (carotid, aortic arch, and renal sensors), central nervous system (CNS) receptors, and hepatic receptors (Table 7-2). The cardiac atria possess the distensibility and the compliance needed to monitor changes in intrathoracic venous volume. An increase in left atrial pressure suppresses the release of antidiuretic hormone (ADH), also called arginine vasopressin (AVP). Atrial distention and a sodium load cause release into the circulation of atrial natriuretic peptide (ANP), a polypeptide normally stored in secretory granules within atrial myocytes. The closely related brain natriuretic peptide (BNP) is stored primarily in ventricular myocardium and is released when ventricular diastolic pressure rises. The atrial-renal reflexes enhance renal sodium and water excretion on sensing of a distended left atrium. The sensitive arterial stretch receptors in the carotid artery, aortic arch, and glomerular afferent arteriole respond to a decrease in arterial pressure. Information from these nerve endings is carried by the vagal and glossopharyngeal nerves to vasomotor centers in the medulla and brainstem. In the normal situation, the prevailing discharge from these receptors exerts a tonic restraining effect on the heart and circulation by inhibiting the sympathetic outflow and augmenting parasympathetic activity. In addition, changes in transmural pressure across the arterial vessels and the atria also influence the secretion of AVP and renin and the release of ANP. Activation of the arterial receptors signals the kidney to retain sodium and water by increases in the sympathetic activity and by increases in vasopressin release. Stimulation of the sympathetic nervous system also enhances the renin-angiotensin-aldosterone system (RAAS). A rise in arterial pressure elicits the opposite response, resulting in decreased catecholamine release and natriuresis. Renal sensing mechanisms include the juxtaglomerular apparatus, which is involved in the generation and release of renin from the kidney. Renin secretion is inversely related to perfusion pressure and directly related to intrarenal tissue pressure. Solute delivery to the macula densa is also an important determinant of renin release because of the tubuloglomerular feedback (TGF) mechanism; an increase in chloride passage through the macula densa results in inhibition of renin release, whereas a decrease in concentration results in enhanced secretion of renin. Renal nerve stimulation through activation of β-adrenergic receptors of the juxtaglomerular apparatus cells directly stimulates renin release. Other receptors reside in the CNS and hepatic circulation but have been less well defined. The stimulation of the effector limb of the ECF volume homeostasis leads to activation of effector mechanisms (Table 7-2). These effector mechanisms aim predominantly at modulation of renal sodium and water excretion to preserve circulatory stability. Sympathetic nerves that originate in the prevertebral celiac and paravertebral ganglia innervate cells of the afferent and efferent arterioles, juxtaglomerular apparatus, and renal tubule. Sympathetic nerves alter renal sodium and water handling by direct and indirect mechanisms.2 Increased nerve stimulation indirectly stimulates proximal tubular sodium reabsorption by altering preglomerular and postglomerular arteriolar tone, thereby influencing filtration fraction. Renal nerves directly stimulate proximal tubular fluid reabsorption through receptors on the basolateral membrane of the proximal convoluted tubule cells. These effects on sodium handling are further amplified by the ability of the sympathetic nerves to stimulate renin release, which leads to the formation of angiotensin II (Ang II) and aldosterone. Renin formation by the juxtaglomerular apparatus increases in response to the aforementioned ECF homeostatic afferent limb stimuli. Renin converts angiotensinogen to angiotensin I, which is then converted to Ang II by the action of angiotensin-converting enzyme (ACE); Ang II can subsequently affect circulatory stability and volume homeostasis. It is an effective vasoconstrictor and modulator of renal sodium handling mechanisms at multiple nephron sites. Ang II preferentially increases the efferent arteriolar tone and thus affects the glomerular filtration rate (GFR) and filtration fraction by altering Starling forces across the glomerulus, which leads to enhanced proximal sodium and water retention. Ang II also augments sympathetic neurotransmission and enhances the TGF mechanism. In addition to these indirect mechanisms, Ang II directly enhances proximal tubular volume reabsorption by activating apical membrane sodium-hydrogen (Na+-H+) exchangers. In addition to a nephron effect, Ang II enhances sodium absorption by stimulating the adrenal gland to secrete aldosterone, which in turn increases sodium reabsorption in the cortical collecting tubule. Prostaglandins are proteins derived from arachidonic acid that modulate renal blood flow and sodium handling. Important renal prostaglandins include PGI2, which mediates baroreceptor (but not beta-adrenergic) stimulation of renin release. PGE2 is stimulated by Ang II and has vasodilatory properties. Increased level of Ang II, AVP, and catecholamines stimulates synthesis of prostaglandins, which in turn act to dilate the renal vasculature, to inhibit sodium and water reabsorption, and further to stimulate renin release. By doing so, renal prostaglandins serve to dampen and counterbalance the physiologic effects of the hormones that elicit their production and so maintain renal function. Inhibition of prostaglandins by nonsteroidal anti-inflammatory drugs (NSAIDs) leads to magnification of the effect of vasoconstricting hormones and unchecked sodium and water retention. The polypeptide AVP is synthesized in supraoptic and paraventricular nuclei of the hypothalamus and is secreted by the posterior pituitary gland. Besides osmotic control of AVP release, there is also a nonosmotic regulatory pathway sensitive to EABV.3 AVP release is suppressed in response to ECF volume overload sensed by increased afferent impulses from arterial baroreceptors and atrial receptors, whereas decreased ECF volume has the opposite effect. AVP release leads to antidiuresis and, in high doses, to systemic vasoconstriction through the V1 receptors.4 The antidiuretic action of AVP results from the effect on the principal cell of the collecting duct through activation of the V2 receptor. AVP increases the synthesis and provokes the insertion of aquaporin 2 water channels into the luminal membrane, thereby allowing water to be reabsorbed down the favorable osmotic gradient. AVP may also lead to enhanced Na+ reabsorption and K+ secretion. AVP appears to have synergistic effects with aldosterone on sodium transport in the cortical collecting duct.5 AVP stimulates potassium secretion by the distal nephron, and this serves to preserve potassium balance during ECF depletion, when circulating levels of vasopressin are high and tubular delivery of sodium and fluid is reduced. Atrial natriuretic peptide is a polypeptide hormone that stimulates diuresis, natriuresis, and vasorelaxation. ANP is primarily synthesized in the cardiac atria and released in response to a rise in atrial distention. ANP augments sodium and water excretion by increasing the GFR, possibly by dilating the afferent arteriole and constricting the efferent arteriole. Furthermore, it inhibits sodium reabsorption in the cortical collecting tubule and inner medullary collecting duct, reduces renin and aldosterone secretion, and opposes the vasoconstrictive effects of Ang II. BNP is another natriuretic hormone that is produced in the cardiac ventricles. It induces natriuretic, endocrine, and hemodynamic responses similar to those induced by ANP.6 Circulating levels of ANP and BNP are elevated in congestive heart failure (CHF) and in cirrhosis with ascites, but not to levels sufficient to prevent edema formation. In addition, in those edematous states, there is resistance to the actions of natriuretic peptides. C-type natriuretic peptide (CNP) is produced by endothelial cells, where it is believed to play a role in the local regulation of vascular tone and blood flow. However, its physiologic significance in the regulation of sodium and water balance in humans is not well defined. Other hormones that contribute to renal sodium handling and ECF volume homeostasis include nitric oxide (NO), endothelin, and the kallikrein-kinin system. NO is an endothelium-derived mediator that has been shown to participate in the natriuretic responses to increases in blood pressure or ECF volume expansion, so-called pressure natriuresis. Endothelins are natriuretic factors, and kinins are potent vasodilator peptides; their physiologic roles are not yet fully defined. Contraction of ECF volume refers to a decrease in ECF volume caused by sodium or water loss exceeding intake. Losses may be renal or extrarenal through the gastrointestinal tract, skin, and lungs or by sequestration in potential spaces in the body (e.g., abdomen, muscle) that are not in hemodynamic equilibrium with the ECF (Table 7-3). The reduction in ECF volume occurs simultaneously from both the interstitial and the intravascular compartment and is determined by whether the volume loss is primarily solute-free water or a combination of sodium and water. The loss of solute-free water has a lesser effect on intravascular volume because of the smaller amount of water present in the ECF compared with the ICF and the free movement of water between fluid compartments. Table 7-3 Major causes of extracellular fluid volume depletion Approximately 3 to 6 liters of fluids and digestive juices are secreted daily throughout the gastrointestinal tract, and most of this fluid is reabsorbed. Vomiting or nasogastric suction may cause volume loss that is usually accompanied by metabolic alkalosis, whereas diarrhea may result in volume depletion that is accompanied by metabolic acidosis. Sweat is typically hypotonic, leading to more water loss than salt loss. Sweat production can be excessive in high ambient temperature or with prolonged exercise in hot, humid climates and may lead to volume depletion. Loss of the skin barrier with superficial burns and exudative skin lesions may lead to significant ECF volume depletion. Body fluid accumulation in potential spaces that are not in hemodynamic equilibrium with the ECF compartment can cause volume depletion. This pathologic accumulation, often called third-space sequestration, includes ascites, hydrothorax, and intestinal obstruction, with fluid collecting in the peritoneal cavity, pleural space, and intestines, respectively, and leading to significant ECF volume loss. Severe pancreatitis may result in retroperitoneal fluid collections. Hemorrhage occurring internally (e.g., from bleeding esophageal varices) or externally (e.g., trauma) may lead to significant volume loss. In the normal individual, about 25,000 mmol of sodium is filtered every day, and a small amount of that quantity is excreted in the urine. The small quantities of sodium excreted in urine relative to the filtered load depend on intact tubular reabsorptive mechanisms to adjust urinary sodium excretion according to the degree needed to maintain ECF homeostasis. Impairment in the integrity of these sodium reabsorptive mechanisms can result in significant sodium deficit and volume depletion. Most of the widely used diuretic medications inhibit specific sites for sodium reabsorption at different segments of the nephron. Diuretics may cause renal sodium wasting, volume contraction, and metabolic acid-base disturbances if abused or inappropriately prescribed. Ingestion of osmotic diuretics results in obligatory renal sodium and water loss, as discussed in detail later. Tubular sodium reabsorption may be disrupted in several genetic disorders that include Bartter syndrome and Gitelman syndrome (see Chapter 49). These autosomal recessive disorders are caused by mutations of sodium transporters that are targets of diuretics or other transporters that are their essential cellular partners. Both syndromes result in sodium wasting, volume contraction, and hypokalemic metabolic alkalosis.7 The tubular defect in Bartter syndrome resembles that of chronic ingestion of loop diuretics. Five variants result from a defect in any of several genes that direct the functioning of transporters in the thick ascending limb of Henle loop. Gitelman syndrome, which is more common in adults, is caused by a defect of sodium chloride (NaCl, salt) reabsorption in the distal tubule. It resembles chronic thiazide diuretic ingestion. Pseudohypoaldosteronism type 1 (PHA1) is a rare inherited disorder characterized by renal sodium wasting and hyperkalemic metabolic acidosis. Acquired tubular disorders that may be accompanied by salt wasting include acute kidney injury (AKI), during the recovery phase of oliguric AKI or urinary obstruction (see Chapters 60 and 71). Mineralocorticoid deficiency and resistance states often lead to sodium wasting. This may occur in the setting of primary adrenal insufficiency (Addison disease) and PHA1. Salt wasting can also be seen in chronic tubular and interstitial renal diseases. Severe hyperglycemia or high levels of blood urea during release of urinary tract obstruction can lead to obligatory renal sodium and water loss secondary to glucosuria or urea diuresis, respectively. Diabetes insipidus (DI) represents a spectrum of diseases resulting from AVP deficiency, causing central DI, or tubular resistance, causing nephrogenic DI, to the actions of AVP. The most common causes of polyuria from nephrogenic DI in adults are chronic lithium ingestion, hypercalcemia, and less frequently, hypokalemia (see Chapter 8). In these disorders the tubular reabsorption of solute-free water is impaired. This generally results in a lesser effect on ECF volume because, in contrast to sodium, there is a relatively smaller amount of the total body water in the ECF compartment compared with the ICF compartment. The spectrum of the clinical manifestations of volume contraction depends on the amount and rate of ECF volume loss as well as on the vascular and renal responses to that loss. An adequate history and physical examination are crucial to elucidate the cause of hypovolemia. Symptoms are usually nonspecific and can range from mild postural symptoms, thirst, muscle cramps, and weakness to drowsiness and disturbed mentation with profound volume loss. Physical examination may reveal tachycardia, cold clammy skin, postural or recumbent hypotension, and reduced urine output, depending on the degree of volume loss (Box 7-1). Reduced jugular venous pressure (JVP) noted at the base of the neck is a useful parameter of volume depletion and may roughly estimate the central venous pressure (CVP). However, an elevated CVP does not exclude hypovolemia in patients with underlying cardiac failure or pulmonary hypertension. The lack of symptoms or discernible physical findings does not preclude volume depletion in an appropriate clinical setting, and hemodynamic monitoring and administration of a fluid challenge may be necessary. Laboratory parameters may assist in defining the underlying causes of volume depletion. Hemoconcentration and increased serum albumin concentration may be seen early with hypovolemia, but anemia or hypoalbuminemia caused by a concomitant disease may confound interpretation of these laboratory values. In healthy individuals, the blood urea nitrogen (BUN)–serum creatinine ratio is approximately 10 mg/dl (40 mmol/l). In volume-contracted states, this ratio may significantly increase because of an associated differential increase in urea reabsorption in the collecting duct. Several clinical conditions affect this ratio. Upper gastrointestinal hemorrhage and administration of corticosteroids increase urea production, and hence the BUN/creatinine ratio increases. Malnutrition and underlying liver disease diminish urea production, and thus the ratio is less helpful to support volume depletion in such clinical settings. Urine osmolality and specific gravity may be elevated in hypovolemic states but may be altered by an underlying renal disease that leads to renal sodium wasting, concomitant intake of diuretics, or a solute diuresis. Hypovolemia normally promotes avid renal sodium reabsorption, resulting in low urine sodium concentration and low fractional excretion of sodium. Urine chloride follows a similar pattern because sodium and chloride are generally reabsorbed together. Volume depletion with metabolic alkalosis (e.g., with vomiting) is an exception because of the need to excrete the excess bicarbonate in conjunction with sodium to maintain electroneutrality; in this case, urine chloride concentration is a better index of sodium avidity. The fractional excretion of sodium (FENa) is calculated by the following formula:
Disorders of Extracellular Volume
Extracellular Fluid Compartment
Total Body Water for 70 kg Man (60% or 42 Liters)
Electrolyte (mmol/l)
Intracellular Water (2/3 or 28 l)
Extracellular Water (1/3 or 14 l)
Interstitial (3/4 or 10.5 l)
Blood (1/4 or 3.5 l)
Na
25
140
K
150
4.5
Mg
0.5
1.0
Ca
0.01
2.4
Cl
2
100
HCO3
6
25
Phos
1.4
1.2
Regulation of Extracellular Fluid Homeostasis
Afferent (Sensor) Limb
Efferent (Effector) Limb
Sympathetic Nervous System
Renin-Angiotensin-Aldosterone System
Prostaglandins
Arginine Vasopressin
Natriuretic Peptides
Other Hormones
Extracellular Fluid Volume Contraction
Extrarenal Causes
Gastrointestinal Losses
Dermal Losses
Third-Space Sequestration
Hemorrhage
Renal Losses
Diuretic Use
Genetic and Acquired Tubular Disorders
Hormonal and Metabolic Disturbances
Renal Water Loss
Clinical Manifestations
Laboratory Tests
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Disorders of Extracellular Volume
Chapter 7