Nigel S. Kanagasundaram, Andrew Lewington
Dialytic Therapies for Drug Overdose and Poisoning
Poisoning and drug overdose, whether intentional or accidental, remains a common medical emergency, accounting for around 140,000 hospital admissions per year in the United Kingdom (nearly 1% of all admissions).1 Most human exposures, however, do not require hospital admission; around 70% of the approximately 2.3 million cases in the United States in 2011 were managed at the site of the incident, in a non–health care facility.2 The spectrum of agents ingested is wide, ranging from overdose of prescription or proprietary drugs to poisoning with nonpharmacologic substances and recreational drugs.
The pattern of toxin ingestion has changed over the years but also varies according to geographic location. Frequently implicated agents in industrialized societies include analgesics (acetaminophen, opioids, salicylates), antidepressants, sedatives, and antipsychotics. Barbiturate poisoning is now less common but was in the past a major contributor. Pesticides remain a frequent cause of poisoning in areas of the developing world.3
Changes in legislation have been instituted in attempts to affect availability of potential toxins; in the United Kingdom, for instance, paraquat was withdrawn from sale in July 2008, although occasional exposures continue as a result of residual stored product.4 U.K. legislation limiting pack size of paracetamol (acetaminophen) in 1998 has been followed by a significant reduction in the number of deaths from paracetamol overdose.5
Data in the United States indicate a low mortality rate—1995 fatalities for 2.3 million episodes in 2011.2 However, poisoning remains a major cause of death in young people.3
The mainstays of management include hemodynamic, respiratory, and other supportive care, prevention of further drug absorption (oral activated charcoal in specific patients), neutralization of drug toxicity (e.g., intravenous N-acetylcysteine after significant acetaminophen overdose or digoxin immune Fab [Digibind] for digitoxicity), and enhancement of drug elimination.
Extracorporeal therapy is one method of achieving poison removal, either by dialysis or by a nondialytic technique such as hemoperfusion. These enhanced elimination techniques are only occasionally needed, with around 2300 patients recorded as requiring extracorporeal therapy in the United States in 2011.2 In that report the treatment was predominantly hemodialysis (HD) with only 14 patients recorded as receiving hemoperfusion, the decline of which is perhaps explained by the increasing rarity of theophylline and barbiturate overdose—historically, the main indications for this technique.
Aspects of the management of poisoning beyond those related to extracorporeal therapies are well covered through resources such as Toxbase in the United Kingdom (www.toxbase.org) and the American Association of Poison Control Centers (www.aapcc.org). Other local and regional poison information services are linked through the website of the European Association of Poisons Centres and Clinical Toxicologists (www.eapcct.org).
Treatment Modalities
Intermittent Hemodialysis and Hemofiltration
Diffusion against a steep concentration gradient, the physical process used in intermittent HD, encourages the rapid removal of the smaller solutes that are the usual agents of overdose. Clearances can be enhanced by increasing dialyzer efficiency (indicated by the KoA, the urea mass transfer area coefficient) or membrane surface area. Larger-solute removal can be enhanced by increasing dialyzer flux when intermittent HD is used (for toxins >500 d and up to 10,000 d, e.g., desferrioxamine [deferoxamine] aminoglycosides) or by switching to hemofiltration (usually applied continuously; see later), in which the physical process of higher volume convection and larger membrane pore size can allow removal of toxins up to approximately 40,000 d.
A range of physical and biologic factors determines whether a given toxin can be eliminated by HD and whether that elimination is actually therapeutic (Box 98-1). In addition to molecular weight, other important physical characteristics relevant to dialyzability are water solubility and the degree of protein binding.
For each individual patient, it should be determined whether HD is expected to contribute significantly to total toxin removal. Dialysis will have a limited impact if the rate of drug removal is significantly faster by endogenous routes. The risks of therapy (e.g., acute transfer to a specialist center, vascular access, anticoagulation) also have to be balanced against the clinical relevance of any gain in drug elimination. If endogenous elimination is minimal—either because these routes are naturally limited or because of toxin-associated attenuation of elimination (e.g., liver or renal failure)—HD can be effective. It is generally accepted that if at least 30% can be added to total body clearance by extracorporeal treatment, its use is justified. This is not a threshold that is readily calculated at the bedside without knowledge of endogenous clearance rates and the expected contribution from exogenous therapy. The summary of product characteristics may help determine the endogenous clearance rates (at least for pharmaceuticals; available from, for instance, www.medicines.org.uk/emc/) but may not account for the impact of disease on specific endogenous routes of elimination. An estimate of the clearances of a variety of solutes of different molecular weights by a dialyzer can be obtained from its product insert. For hemofiltration (see the later discussion of continuous renal replacement therapy [CRRT]), an estimate of clearance can be provided by the ultrafiltration rate.
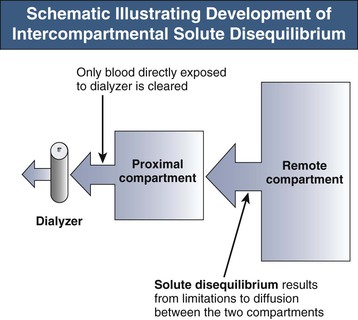
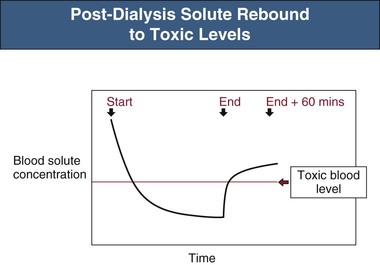
The efficacy of toxin removal is also influenced by its theoretical volume of distribution (VD). Substances confined to the bloodstream will have a low VD (approximately 0.07 l/kg body weight); those distributed in the extracellular space, a VD of approximately 0.2 l/kg; and those confined to total body water, approximately 0.6 l/kg. Higher distribution volumes are, however, not uncommonly found in those substances with avid tissue binding or sequestration. As VD increases, more solute must be removed for achievement of a particular blood level. The ideal solute would therefore have a low distribution volume, which would also be a single, well-mixed compartment that was directly accessible by the dialysis process. However, as shown in Figure 98-1, solute is often distributed across at least one remote body compartment that is not directly accessible during HD. If there is any resistance to solute movement between the accessible proximal and the remote compartments, disequilibrium will develop over the course of the dialysis session, reducing the overall efficiency of toxin removal. This may be significant, as in the case of lithium, and will manifest as a large postdialysis rebound in blood levels as solute re-equilibrates from the remote compartment. A significant solute rebound to a potentially toxic level may be missed if the immediate postdialysis blood level is relied on as a measure of elimination (Fig. 98-2).
Extending the HD session beyond 4 hours can to some extent ameliorate rebound, but intermittent HD is an inherently inefficient process that depends on the solute concentration presented to the dialyzer. Most solute removal occurs at the start of dialysis. Any gains in solute removal will be disproportionately low in comparison to the increases in dialysis time. An alternative or adjunctive solution is to increase dialysis session frequency.
Compartmentalization need not preclude HD-related toxin removal, but the closer its distribution is to a single compartment, the easier that removal becomes.
In summary, intermittent HD is usually the first-choice extracorporeal modality because of its common availability, the rapidity of toxin removal, and the low molecular weight of the common agents of poisoning. The role of other renal replacement modalities is less clear because of a lack of published data.
Peritoneal Dialysis
Peritoneal dialysis is rarely used in the treatment of poisoning because of the comparatively slow rate of clearance, the risks associated with acute peritoneal dialysis catheter insertion, and the widespread availability of extracorporeal techniques (at least in the industrialized world). It may have a role in the treatment of poisoning in children because the lower clearance may be sufficient for their smaller solute distribution volumes. There may, in addition, be technical challenges making HD less satisfactory, especially in the very young.
Continuous Renal Replacement Therapy
Continuous renal replacement therapy may be used when intermittent HD is not immediately available or when more rapid solute removal would be compromised by significant intercompartmental disequilibrium. For small-solute clearances, continuous hemofiltration and continuous HD have near kinetic equivalence. Full saturation of dialysate effluent in continuous HD, because of its slow flow rates, gives it a small-solute concentration similar to both the ultrafiltrate from hemofiltration and plasma water as it leaves the hollow-fiber device. CRRT gives better longer-term solute clearances (over the course of several days) but does not provide the rapidity of elimination afforded by intermittent HD when minimizing toxin exposure is a high priority. Delivered small-solute clearances of CRRT can be maximized by combining techniques in the form of continuous hemodiafiltration. If it is logistically possible, an ideal combination may be initial use of intermittent HD for rapid reduction of toxin levels, with continuous therapy then being applied to ameliorate any postdialysis rebound when this is predicted. Although combining some of the advantages of both continuous and intermittent techniques, the role for hybrid modalities, such as sustained low-efficiency dialysis (SLED), requires further evaluation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree