Mark R. Marshall, Luis A. Juncos
Dialytic Management of Acute Kidney Injury and Intensive Care Unit Nephrology
Intensive care unit (ICU) nephrology can be defined as a subspecialty that focuses on abnormalities of fluid, electrolyte, and pH homeostasis in ICU patients, and the prevention and management of functional renal impairment relative to physiologic demand. This chapter describes agreed-on best practices with respect to acute renal replacement therapy (ARRT) and provides strategies for avoiding common treatment-related complications. The chapter uses appropriate clinical practice guidelines as starting points for discussion and summarizes their recommendations (Box 74-1).1–8
When the Acute Kidney Injury Network (AKIN) criteria are applied, approximately 40% of ICU patients develop evidence of acute kidney injury (AKI), an independent risk factor for death. The nondialytic therapy of AKI is discussed in Chapter 73. Approximately 5% of ICU patients require ARRT, and mortality in this population is improving over time despite a higher degree of illness severity.9 Death attributable to AKI appears to result from nonresolving infection, hemorrhage, or nonresolving shock, despite optimal care. Such conditions may therefore be considered a systemic “acute uremic syndrome” that is specific to AKI and a possible target for modulation with ARRT—analogous to the traditional uremic syndrome in the setting of maintenance dialysis.
Organizational Aspects of Acute Renal Replacement Therapy Programs
Intensive care units can be described as open (patient care remains under the attending physician of record), closed (patient care is transferred to an intensivist), or comanaged (an open ICU, as defined earlier, in which patients receive mandatory consultation from an intensivist). Most ICUs in the United States are open, whereas most in Australia and New Zealand are closed. Those in Europe are approximately equally split. When AKI and ARRT are considered, advantages of intensivist-based management include immediate availability of service and decreased fragmentation of care. This model of care is supported by ecologic studies that suggest improved overall patient outcomes in health care systems with closed ICUs. Alternatively, advantages of nephrology-based management include greater understanding of the dialysis process and underlying AKI. This model of care is supported by studies that show improved outcomes in ICU patients with AKI associated with earlier referral to nephrology. All of these studies have disparate definitions and analyses that make interpretation difficult. Clinical governance over ARRT is therefore likely to remain contentious, although the expertise of staff providing care probably influences patient outcomes more than the specialty to which they belong. Specific training in ICU nephrology and exposure to ARRT is inadequate in many critical care and nephrology training schemes and should be a component of both core curricula.
In many parts of the world, ICU nursing staff members deliver all modalities of ARRT; in other countries, support from nephrology staff is required. As machinery platforms become universal for continuous renal replacement therapy (CRRT) and intermittent hemodialysis (HD), it is likely that ICU expertise in all modalities will grow, provided in-service education and support are adequate to develop and maintain the skill base.
Overview of Acute Renal Replacement Therapies
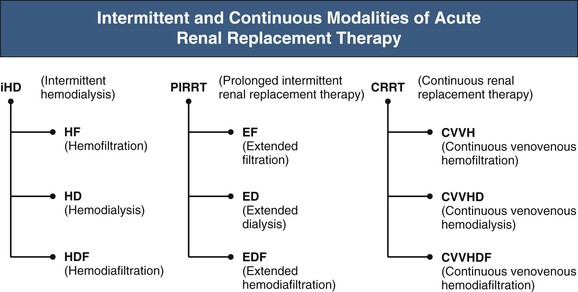
The main modalities of ARRT are acute intermittent HD and its variants, CRRT, and acute peritoneal dialysis (PD). Acute intermittent HD and CRRT are most popular, although practice patterns vary regionally because of cost, availability of technology, and reimbursement policies. Recently, intermittent HD has undergone resurgence through variants that provide slower fluid removal over longer periods of time, resulting in improved hemodynamic stability and increased solute clearance. These variants are most commonly referred to as sustained low-efficiency dialysis (SLED). Collectively, however, they are best referred to with the umbrella term prolonged intermittent renal replacement therapy (PIRRT), a term more aligned to nomenclature endorsed by the Acute Dialysis Quality Initiative (www.adqi.net) (Fig. 74-1). Acute PD is mostly used in the developed world for pediatric patients, and it is not considered further in this chapter.
Therapeutic goals for ARRT are not well defined. The usual minimum recommendation is to correct acidosis or hyperkalemia, refractory hypervolemia, and uremic features such as pericarditis or coma. Serum electrolyte and bicarbonate concentrations should be maintained in the normal range. Although specific laboratory thresholds for starting and stopping therapy are unknown, dialysis dose should be measured and adjusted to meet minimum targets, which are discussed later. It is important to note that the process of ARRT itself should not jeopardize the patient by exacerbating hemodynamic instability, increasing end-organ damage, or delaying renal recovery.
Determining goals with respect to a patient’s extracellular volume status is not straightforward. Assessment itself is difficult; physical signs such as jugular venous distention are generally not informative, especially for mechanically ventilated patients. Moreover, baseline values of central venous pressure, pulmonary capillary wedge pressure, and echocardiographic left ventricular diastolic dimensions may be inaccurate surrogates for intravascular volume status, especially for patients with sepsis. An alternative approach is to use the effect of therapeutic maneuvers such as fluid challenge on blood pressure, stroke volume, or vena cava collapsibility. Even after fluid status has been adequately assessed, determining the therapeutic goal is also difficult; patients with extracellular fluid excess in the absence of intravascular hypervolemia may benefit from fluid removal if they develop abdominal compartment syndrome, impairment of lung compliance and oxygenation, or poor wound healing. In particular, patients with acute lung injury require a shorter period of ventilation with less fluid loading (guided by central filling pressures).
Variations of ARRT have been used to enhance removal of cytokines. Cytokines are medium (300 to 12,000 d) and large (>12,000 d) molecules that are essential in modulating the immune response. However, their production is increased with acute illness, and their clearance decreased during AKI, resulting in excessive levels that have cardiodepressant, vasodilatory, and immunosuppressive properties.10 Consequently, this has sparked interest in enhancing cytokine clearance during acute illness or AKI. Differing degrees of cytokine removal can be achieved through the use of high-flux or super-high-flux membranes (molecular weight cutoff of approximately 60 to 150 kd), bioadsorption and coupled plasma filtration–adsorption filters, and high levels of convective clearance. The last is achieved by ultrafiltering large amounts of plasma through a standard hemofilter and replacing the ultrafiltrate with a physiologic replacement fluid, thus diluting the remaining solutes in the body. It should be noted, however, that ARRT will remove both proinflammatory and anti-inflammatory cytokines, with potential to inadvertently exacerbate the inflammatory milieu. Notwithstanding, a promising technique is high-volume hemofiltration, supported by observations of improved outcomes in ICU patients with sepsis with higher doses of hemofiltration (≥45 ml/kg/h), and improved hemodynamic stability with even higher doses (60 to 100 ml/kg/h) applied either as a “pulse” or continuous maneuver. At present, only preliminary outcome data support the approach, although clinical trials are under way.11
Timing of ARRT initiation is controversial. Proponents of early initiation argue that it is in the patients’ interest to prevent rather than treat the acute uremic syndrome and recommend initiation once kidney injury or failure is present. Indeed, this view is supported by observational studies suggesting that earlier initiation might achieve better outcomes (none suggest more harm). However, there is no high-quality evidence, and the single clinical trial designed to answer the question was inadequately powered.12 Currently, timing of initiation is variable; a large multinational cohort study showed ARRT was initiated when the median (interquartile range [IQR]) serum creatinine and urine output were 309 (202 to 442) µmol/l and 576 (192 to 1272) ml/day, respectively.13
Intermittent Acute Renal Replacement Therapy
Techniques for Acute Intermittent Hemodialysis
Acute intermittent HD is categorized according to hemodialyzer membrane and mechanism of solute removal. High-flux membranes allow greater convective removal of middle and larger solutes, but limited clinical data do not show obvious advantages in the ICU setting. Biocompatibility is a membrane characteristic that includes a low capacity for activating complement and leukocytes. After complement activation, there is stasis of leukocytes in the lungs, renal parenchyma, and other organs and the release of products of leukocyte activation. Although studies have been inconsistent, the use of biocompatible membranes should in principle favorably affect mortality and recovery of renal function in ICU patients with AKI, and are recommended.
Hemodiafiltration (HDF) is usually performed in the ICU as a continuous modality. However, acute intermittent HDF can be performed with use of sterile replacement fluid generated from ultrapure dialysate (“online”), which is infused directly into the extracorporeal blood circuit. As with high-flux dialysis, limited clinical data do not show obvious advantages.
Dialysate for intermittent ARRT can either be delivered by a batch system or generated online by a single-pass system. The latter uses concentrate and tap water that is purified by reverse osmosis in a central water purification plant or portable unit. Most ICUs do not have a central plant, although this is increasingly common in units in which on-line HDF is performed. There is concern about the possibility of backfiltration of bacterial contaminants, specifically endotoxin, which could exacerbate cytokine-mediated injury. At this time, the reference standards for water purity in the ICU setting are the same as those for the end-stage renal disease (ESRD) setting (www.iso.org; see Box 74-1). Online replacement fluid for HDF is sterilized with ultrafilters in the dialysate pathway and does not differ from commercial preparations in terms of microbial counts, endotoxin concentration, and cytokine-inducing activity. Sterile dialysate is suggested by some for all acute intermittent HD, although there are insufficient data to support a strong recommendation.
Strategies to Reduce Intradialytic Hemodynamic Instability During Intermittent Hemodialysis
Intradialytic hypotension is detrimental for end-organ function and recovery. Fresh ischemic lesions in kidney biopsy specimens can be found in patients on ARRT of longer than 3 weeks’ duration. The relatively high ultrafiltration rate (UFR) with acute intermittent HD often leads to intradialytic hypotension, which reduces residual renal function. Increasing the frequency and treatment time of intermittent HD minimizes ultrafiltration goals and is the most effective measure to minimize intradialytic hypotension. Certain technical features on dialysis machinery are also helpful. Hemodynamic stability is facilitated by precise and predictable fluid removal, especially when the fluid removal is greater than that required for the restoration of euvolemia, such as for HDF. Hence, machines with computerized flow or volumetric ultrafiltration control are preferred.
Bicarbonate-buffered dialysate should be used routinely in critically ill AKI patients. It is associated with less hypotension than acetate dialysate, which has a peripheral vasodilating and myocardial depressant effect.
Hemodynamic stability is also facilitated by sodium profiling during intermittent HD. The rapid reduction in serum osmolality with intermittent HD promotes water movement into cells, thus reducing effective circulating volume. Dialysate sodium concentration can vary from approximately 130 to 150 mmol/l. The default for intermittent HD and PIRRT is approximately 145 mmol/l and avoids changes in sodium mass balance that can lead to marked fluid shifts and therefore intradialytic hypotension. Sodium profiling further ameliorates this process by inducing water flux into the vascular compartment, although an alternative and simpler approach of use of a high-sodium dialysate (e.g., 145 to 150 mmol/l) may also work and needs to be tested. A randomized study showed that intermittent HD with sodium profiling (starting at 160 mmol/l, reducing to 140 mmol/l) combined with ultrafiltration profiling (50% of ultrafiltration volume removed in first third of treatment) improved hemodynamic stability.14 Profiling seems to be effective, although it should be used carefully in patients with dysnatremias, in whom serum sodium concentrations should be corrected slowly to minimize the risk of neurologic complications.
Online blood temperature and blood volume monitoring involves biofeedback systems that automatically adjust intermittent HD operating parameters. Blood volume monitoring adjusts UFR and dialysate sodium content in response to a fall in circulating blood volume, and blood temperature monitoring maintains blood temperature at a target value by controlling thermal transfer to and from dialysate to avoid vasodilation and decreased vascular resistance. Although helpful in the ESRD setting, neither technique has been shown to prevent intradialytic hypotension in the ICU setting.15,16 The likely reasons pertain to different causes and compensatory mechanisms for hypotension that probably differ in the two settings.17
High dialysate calcium (1.75 mmol/l) has been used to improve hemodynamic stability during intermittent HD in ESRD patients with cardiomyopathy. This technique is limited by the development of hypercalcemia, however, and has not been studied in the ICU setting.
A number of early observational studies demonstrated less intradialytic hypotension during intermittent HDF in the ESRD setting, although prospective controlled trials, such as the Convective Transport Study (CONTRAST), have not supported this finding.18 It is unlikely that intermittent HDF reduces intradialytic hypotension in critically ill AKI patients.
Extensive cumulative clinical experience shows that lower-efficiency modalities of ARRT provide better hemodynamic stability because of slower fluid and solute removal. This is supported by meta-analyses showing better preservation of blood pressure and lower vasopressor requirements in those treated with CRRT rather than intermittent HD.1,19 Several prospective clinical trials and many observational studies have also shown comparable hemodynamic stability between CRRT and PIRRT.20–23 Lower-efficiency prescriptions of PIRRT and CRRT are therefore suitable for ameliorating intradialytic hypotension, and the first choice of ARRT modality for hemodynamically unstable patients.
Prolonged Intermittent Renal Replacement Therapy
Prolonged intermittent renal replacement therapy uses standard intermittent HD equipment and consumables, but with lower solute clearances and UFR maintained for prolonged periods of time.24 Typically, treatment duration is 6 to 18 hours. Dialysate flow rate (Qd) is usually 200 to 300 ml/min, and urea clearances are therefore lower and higher than in intermittent HD and CRRT, respectively, allowing for scheduled downtime without compromising dialysis dose. With longer treatments, phosphate replacement may be required at 0.1 to 0.2 mmol/kg or by adding 30 to 45 ml of a bowel preparation containing sodium dihydrogen phosphate dihydrate and disodium phosphate (e.g., Fleet Phospho-Soda) to dialysate. In addition, dietary protein should be supplemented by 0.2 g of PIRRT per kilogram per day. A prescription algorithm is shown in Figure 74-2.
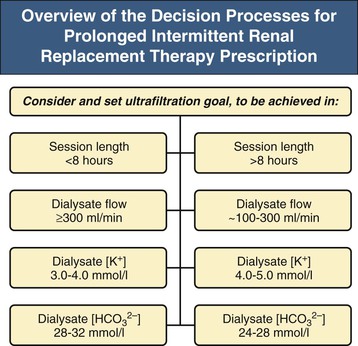
Prolonged intermittent renal replacement therapy provides a high dose of dialysis with minimal urea disequilibrium, excellent control of electrolytes, and good tolerance to ultrafiltration.20 PIRRT is usually delivered as a diffusive therapy, although there is increasing experience with combined diffusive and convective clearance.
Dosage of Acute Intermittent Renal Replacement Therapy
The relationship between small-solute clearance and the outcomes of critically ill AKI patients is now established. A key study from 20 years ago showed that delivered single-pool Kt/V (spKt/V) above 1.0 per intermittent HD treatment was associated with improved survival in patients with intermediate illness severity, although the study did not relate outcomes to frequency of treatments.25 Most recently, a well-conceived and executed prospective, randomized controlled trial showed that delivered spKt/V of 1.2 to 1.4 per intermittent HD or PIRRT treatment five to six times per week did not improve survival compared with this dose thrice weekly.26 There are consistent data restricted to PIRRT from the Hannover Dialysis Outcome Study, a smaller but well-executed randomized clinical trial (see later).27
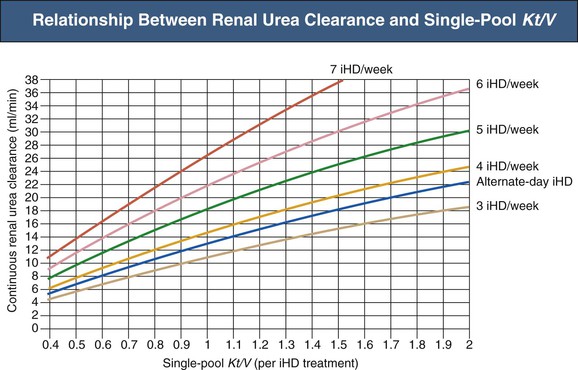
Optimal intermittent HD dose therefore appears to be related to small-solute clearance, although there appears to be a dose above which survival becomes dose independent. The minimum recommended intermittent HD and PIRRT dose in ICU patients with AKI is a delivered spKt/V of at least 1.3 per treatment at least thrice weekly.1 Because routine dialysis regimens used in the United States in this setting have been reported to deliver a dose of spKt/V that is less than 1, routine measurement of dose should be undertaken to guide appropriate adjustment of operating parameters as summarized in Box 74-2. If this dosage target cannot be achieved, dose should be maintained as high as possible and treatment frequency increased. The required number of treatments per week and administration interval can be established from the nomogram in Figure 74-3, expressing combinations of intermittent HD dose and treatment frequency as a continuous small-solute clearance (expressed as the corrected equivalent renal urea clearance [EKRc]), aiming for a value of 13 ml/min or higher.28,29 This expression of dose is useful to frame interpretation of outcomes in the Hannover Dialysis Outcome Study: patients treated with daily PIRRT to keep plasma urea at 11.3 ± 4 mmol/l (EKRc equal to 20 ml/min, assuming urea generation of 20 mg/min) had outcomes indistinguishable from those of patients treated to keep plasma urea at 19 ± 6 mmol/l (EKRc equal to 13 ml/min).27 In both arms, the doses expressed as EKRc were above the value that defines adequate intermittent HD delivering an spKt/V of 1.3 or higher at least thrice weekly (EKRc ≥ 13 ml/min in Fig. 74-3).
Continuous Renal Replacement Therapy
Continuous renal replacement therapy involves the application of lower UFR and solute clearances for substantial periods every day. The lower UFR provides comparatively better hemodynamic stability than intermittent HD, especially during ultrafiltration of large obligatory fluid loads, and the lower solute clearances result in single-pool solute kinetics despite disparities between regional blood flows resulting from pressor use. The longer treatment duration results in better and more consistent control of uremic solutes, especially for severely catabolic patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree