3
Diagnostic Concerns and Evaluation of the Andropause in Aging Males
Case History
A 50-year-old man who has a long history of depression, and also suffers from sexual dysfunction. He has a past medical history of hypertension, coronary artery disease, and hypercholesterolemia. He has also attended Alcoholic Anonymous meetings. He is currently on Prozac, Lipitor, atenolol, several vitamins, and various herbal preparations thought to increase sex drive. He reports that he has difficulty with erections. His wife complains that he has lost the desire for sex. On direct questioning, he admits that he has lost the ability for early-morning erections. He also feels easily tired, and unable to stay awake right after dinner. For the past 3 months he has also complained of periodic night sweats, and he would wake up in the morning drenched. Examination revealed that he weighed 225 pounds, his blood pressure (BP) was 135/100 mm Hg, and he had loss of hair in the armpits. His testicular size was compared with an orchidometer and his left testes had a reduced volume compared with the right. He scored 8/10 on the Androgen Decline in Aging Males (ADAM) questionnaire. His laboratory results revealed that the 8 a.m. total testosterone was 148ng/dL (range 260–1000), bioavailable testosterone was 88ng/dL (range 84–403), estradiol 26pg/dL, luteinizing hormone (LH) 1.8 mIU/mL, prolactin 19.2ng/dL, prostate-specific antigen (PSA) 1.5ng/dL, leptin 19.2ng/dl (range 1.2–9.5). The course of treatment of the patient is discussed at the end of the chapter.
Is There Indeed an Andropause and If So, Can There Be Symptoms?
It is not unusual for physicians to treat men in their 40s and older who complain of loss of libido, erectile dysfunction, fatigue, and depression. Psychological problems and medical illness are often confounders to the andropause. Knowledge of the patient’s history and a careful examination combined with laboratory tests are keys to an accurate diagnosis of symptomatic hypogonadism, or the andropause syndrome.1
Androgens are a group of hormones that include testosterone, dehydroepiandrosterone (DHEA), androstenedione, 3 alpha-androstanediol glucuronide (AAG), and others. It is a misnomer to classify them as “male hormones” as they are present in both males and females, albeit in different amounts. There is undeniable evidence that aging results in a lowering of androgens. When total testosterone is measured, 20% of men above 55 years are hypogonadal.2,3 When bioavailable testosterone is measured, however, 50% of men above 50 years are defined as hypogonadal.4 Ninety-eight percent of circulating testosterone is bound to plasma proteins; the remaining 2% of free testosterone is responsible for biological activity. Approximately 40% of the bound testosterone is bound to sex hormone–binding globulin (SHBG). The rest is weakly bound to albumin and is readily available to tissue when needed. Bioavailable testosterone includes free testosterone and that loosely bound to albumin.5
As the decline in androgens is gradual, the alternative term of androgen decline in aging males (ADAM) has been used. Partial androgen decline in aging males (PADAM) has also been suggested because the androgen deficiency in older men is generally moderate and not a complete deficiency. There is often confusion that andropause is a symptomatic state. It must be emphasized that like menopause, there could be the presence or absence of symptoms. Transitory symptoms can include changes in mood and sexuality. The long-term effects of hypogonadism can result in osteoporosis, muscle atrophy, and cognitive changes. Symptomatic hypogonadism is sometimes referred to as the “andropause syndrome.”6
| Ahypogonadal state in older males resulting from gradual partial androgen deficiency, alternately termed androgen decline in aging males (ADAM) or partial androgen decline in aging males (PADAM) |
| Decreased sensitivity to androgens in target organs (thus absolute serum levels of testosterone can be misleading) |
| Symptoms include decreased energy and well-being, changes in sexual function: symptomatic andropause or the “andropause syndrome” |
| In the long term, androgen deprivation can affect bone, muscle, lipids, and cognition |
Symptoms can develop with the andropause syndrome. Our previous study in 302 male subjects revealed that loss of libido and erectile dysfunction (46%), fatigue (41%), and memory loss (36%) were dominant symptoms, in that order.1 The correlation of symptoms to levels of testosterone is very variable and is the subject of ongoing investigation by my team. Most laboratories give a normal range of 260 to 1000ng/dL for total testosterone, 50 to 210pg/dL for free testosterone, and 66 to 417ng/dL for bioavailable testosterone.7 The International System of Units (SI) conversion factor is ∼35. The range often is not age adjusted and poses dilemmas for physicians. Patients can have low-normal levels and yet display symptoms, which are reversed after androgen supplementation. This suggests the possibility of “relative hypogonadism” in which levels are appropriate for each individual.8 Another dilemma is that testosterone levels can vary in the course of a day. Frequent sampling of testosterone in a study of 20 normal men revealed ranges from 105 to 1316ng/dL between subjects.9 Table 3–1 summarizes definitional aspects of andropause.
Andropause and Menopause: Similarities and Contrasts
Andropause refers to a lowered state of androgens. Total testosterone declines ∼1.6% per year, free testosterone decreases 2% per year, and bioavailable testosterone decreases 2 to 3% per year between the age of 40 and 70 years. On the other hand, SHBG increases 1.6% per year (Fig. 3–1).10
Testosterone declines gradually in the andropause. In contrast, menopause usually has a narrower window of 5 to 10 years, with the ultimate shutdown of the ovaries. The production of sperm in males is primarily regulated by LH on Leydig cells in the testis. Testosterone has a supplementary role in regulating spermatogenesis. Fertility in males may not be affected in the andropause, although the numbers of sperm with normal motility and morphology can be altered.11 In contrast, menopause brings about a cessation of reproductive capability.

FIGURE 3-1. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. (Adapted from Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab 2002; 87:589–598, with permission.)
It is important to realize that long-term deprivation of androgens and estrogens in both males and females leads to similar outcomes: osteoporosis, muscle loss, and cognitive changes. The precise mechanism whereby androgens affect bone and cognition is unclear, and it is postulated that the effect is both from testosterone itself and the conversion to estradiol.12–14
Hypogonadism or Low Testosterone Results from Aging
Hypogonadism or low testosterone level with aging is the result of both primary gonadal failure and hypothalamic-pituitary failure. Primary hypogonadism refers to a decrease in the numbers of Leydig cells, reduction of testosterone production, and decreased secretion of testosterone in response to the stimulation of human chorionic gonadotropin. The decline in testosterone levels with aging is associated with increase in follicle-stimulating hormone (FSH) and, to a lesser extent, LH. Low testosterone level with abnormal LH pulse suggests an age-associated impairment of hypothalamic gonadotropin-releasing hormone secretion.15,16
Physiology and Regulation of Androgens
In the normal male, the testes produce 95% of androgens, namely testosterone. About 5 to 10 mg of testosterone is produced on a daily basis by the testes. The adrenals produce the rest of the androgens mainly in the form of DHEA. The pituitary gland secretes LH and regulates the production of testosterone in Leydig cells from cholesterol. FSH mainly affects spermatogenesis. In turn testosterone is metabolized to dihydrotestosterone (DHT) by 5α-reductase and aromatized to estradiol by aromatase. DHT is linked to prostate hypertrophy and male alopecia (Fig. 3–2).17
Influence of Age and Obesity on Testosterone and Sex Hormone-Binding Globulin
In the uterus during the first trimester, fetal testes produce testosterone. Levels peak at about age 20 years, and there is a gradual decline thereafter. Total testosterone declines at the rate of 110 ng/dL, typically after 40 years of age.18 Bioavailable testosterone declines much more dramatically especially with aging. Bioavailable testosterone is thought to be the active component of androgen acting directly at the cellular level. It consists of free testosterone and that loosely bound to albumin. SHBG binding increases with the aging process and hence decreases available free testosterone.19,20 Overall, the impact of aging on testosterone is negative. Obesity in males is accompanied by a significant decrease in testosterone levels. In moderate obesity, free testosterone levels can be apparently “normal” because of the decrease of SHBG. In massively obese males, there is “real” hypogonadotrophic hypogonadism with deceased free testosterone levels.21
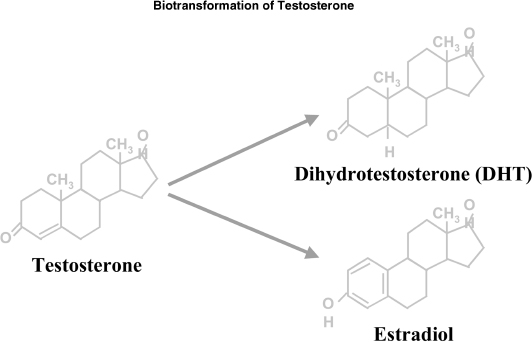
FIGURE 3-2. Biotransformation of testosterone.
Clinical Assessment, Screening Questionnaires, and Confounding Factors for Diagnosis of Andropause
It is important for the physician to take a careful history and to do a physical examination when assessing a patient for hypogonadism. The physician should inquire about symptoms of loss of libido and distinguish it from erectile dysfunction. A loss of early-morning erection can be indicative of hypogonadism. Stress and chronic illness can depress testosterone levels, and so can medications including cimetidine, digoxin, and spironolactone. Diabetes, insulin resistance, and obesity have been associated with hypogonadism.2,11,22 As such, one should look for symptoms and signs associated with diabetes. A history of chronic alcoholism must be ascertained as it can suppress the production of androgens. Rare conditions can be associated with hypogonadism including Prader-Willi syndrome and Klinefelter’s syndrome, as well as Kallmann’s syndrome and they have to be excluded in the clinical examination. The physical examination should include weight, body mass index, waist/hip ratio, and a measurement of body fat.23 The skin should be examined for evidence of hyperestrogenism such as spider telangiectasia. The face, axilla, and groin should be inspected for hair loss. Testicular size can be measured using an orchidometer. A prostate examination should also be done as part of the screening.
Screening tools like the ADAM questionnaire (Table 3–2) can be helpful.24 Screening for depression can be done with the Geriatric Depression Scale.25 The Folstein Mini-Mental State Examination can be used to screen for cognitive problems.26 Unfortunately, memory loss in the andropause can be very subtle, and more sophisticated neuropsychological tools may sometimes be needed.27 There are several confounders to the andropause syndrome, and these are summarized in Table 3–3.
| Do you have a decrease in libido (sex drive)? |
| Do you have a lack of energy? |
| Do you have a decrease in strength and/or endurance? |
| Have you lost height? |
| Have you noticed a decreased enjoyment in life? |
| Are you sad and/or grumpy? |
| Are your erections less strong? |
| During sexual intercourse, has it been more difficult to maintain erection to completion of intercourse? |
| Are you falling asleep after dinner? |
| Has there been a recent deterioration in your work performance? |
| Chronic illnesses (e.g., diabetes, chronic renal failure, cirrhosis) |
| Depression |
| Decrease in albumin from alcohol and poor nutrition |
| Acute stress (e.g., surgery, severe burn, acute myocardial infarction) |
| Medications (e.g., cimetidine, spironolactone, antidepressants) |
| Other endocrinopathies including hypothyroidism, Cushing’s syndrome |
| Hypothalamic-pituitary tumor, hemochromatosis |
| Circadian rhythm of testosterone |
| Kallmann’s syndrome, Klinefelter’s syndrome |
Laboratory Assessments for Diagnosis of Andropause
Laboratory assessments should be used only in conjunction with a good history and examination before deciding that the patient has the andropause syndrome. Reliance on laboratory tests alone can often lead to misdiagnosis. Bioavailable testosterone is preferred in older patients above 65 years because of alterations in binding with SHBG. Most laboratories do not offer an actual free testosterone and calculate free testosterone based on a formula after measuring total testosterone. Radioimmunoassay remains the most common method to assess testosterone levels. Saliva testing for testosterone is a novel approach, allowing convenience for the patient. If done correctly, it has a good correlation to free testosterone levels.28 Bioavailable testosterone levels <60ng/dL or total testosterone levels <300ng/dL with consistent clinical symptoms and signs are diagnostic of androgen deficiency.3,4 The physician must also be aware that testosterone is secreted in spurts, and levels are highest after awakening. However, the daily fluctuation in serum testosterone levels is attenuated in older men.
If the initial testosterone level is low, the evaluation should determine whether there are reversible causes of low testosterone. LH and FSH should also be measured. Some men in andropause demonstrate a slight rise in LH. However, the rise is modest in comparison to the menopause. Prolactin measurements are useful to screen for rare causes of hypogonadism secondary to prolactinoma. As there may be overlapping conditions mimicking the andropause syndrome, it would be prudent to measure TSH and cortisol levels if indicated. Routine laboratory tests, for example, complete blood count, liver function, renal function, and PSA, are also indicated. In geriatric patients whose nutrition may pose a problem, a measurement of zinc levels may be useful. Zinc has antiaromatase action and can be used to treat hypogonadism.29 A repeated serum testosterone level should always be obtained to confirm androgen deficiency before starting testosterone replacement. If the initial free or bioavailable testosterone is within normal range in a man with symptoms and signs of androgen deficiency, the patient’s clinical status and testosterone levels should be monitored on follow-up visits.30
Discussion of Case History
The patient was diagnosed to be hypogonadal based on his symptoms and laboratory results. It was hypothesized that his sexual dysfunction, fatigue, and depressed moods were related to his hypogonadal state, resulting in the andropause syndrome.
A therapeutic trial of compounded testosterone 5 mg with 100 mg zinc in a topical gel vehicle was prepared for him. Zinc was added as it has some antiaromatase activity and may decrease his conversion of testosterone to estradiol. As obesity results in increased aromatase activity, he was put on an exercise and diet regimen. The exercise advised was a combination of strength training and aerobics four times a week. He was advised to stop drinking completely. After 3 months of follow-up, the patient returned reporting significant improvement in quality of life. His sexuality had returned, he felt less tired, and his mood had improved, so that it was decided that his Prozac should be discontinued. Prozac in itself can contribute to sexual dysfunction. His blood pressure was better controlled, and so the atenolol was stopped completely. Part of this improvement was because of his 20-pound weight loss, and perhaps the vasodilatory effects of testosterone. Testosterone has also been known to modulate leptin, thereby reducing weight by decreasing satiety. The total testosterone remeasured was 275 ng/dL, and PSA was 1.5 ng/dL. In retrospect, the patient wonders if his depressed moods were related to his hypogonadal state, but it is difficult to ascertain, and the patient will be followed up, and his mood assessed. The lesson learned in this case is that if androgen supplementation is combined with lifestyle modifications, the outcome is usually better in the long run.
Conclusion and Key Points
• The physician should have knowledge of the multiple factors that can affect testosterone levels.
• Some of the factors that are associated with the andropause syndrome results from lifestyle habits. As such, counseling may play a big role in these patients. Weight loss may potentially reverse hypogonadism in some obese men, although this is an area for further research. Exercise, in particular weight training, may even increase testosterone in older men.31
• There has been overreliance on laboratory assessment of the andropause syndrome, and as such many patients are underdiagnosed or even over-diagnosed at times. The physician must be aware that the testosterone level is not static and that it changes with time. Listening to the patient carefully is the key to a successful treatment plan.
REFERENCES
1. Tan RS, Philip P. Perception and risk factors for the andropause. Arch Androl 1999;43:97–103
2. Smith KW, Feldman HA, McKinlay JB. Construction and field validation of a self-administered screener for testosterone deficiency (hypogonadism) in aging men. Clin Endocrinol (Oxf) 2000;53:703–711
3. Tenover JS. Androgen administration to aging men. Endocrinol Metab Clin North Am 1994;23:877–892
4. Sih R, Morley JE, Kaiser FE, et al. Testosterone replacement in older hypogonadal men: a 12-month randomized controlled trial. J Clin Endocrinol Metab 1997;82:1661–1667
5. Basaria S, Dobs AS. Hypogonadism and androgen replacement therapy in elderly men. Am J Med 2001;110:563–572
6. Morales A, Heaton JP, Carson CC. Andropause: a misnomer for a true clinical entity. J Urol 2000;163:705–712
7. DeGroot LJ, Jameson JL. Endocrinology. 4th ed. Philadelphia: WB Saunders; 2001
8. Tan RS. Andropause: introducing the concept of “relative hypogonadism” in aging males. Int J Impot Res 2002;14:319
9. Spratt DI, O’Dea LS, Crowley WF, et al. Neuroendocrine-gonadal axis in men: frequent sampling of LH, FSH and testosterone. Am J Physiol 1988;254:658–666
10. Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab 2002;87:589–598
11. Gallardo E, Guanes PP, Simon C, et al. Effect of age on sperm fertility potential: oocyte donation as a model. Fertil Steril 1996;66:260–264
12. Siemenda CW, Loncope C, Zhou L, et al. Sex steroid and bone mass in older men: possible associations with serum estrogens and negative association with androgens. J Clin Invest 1997; 100:1755–1759
13. Pietschmann P, Kudlacek S, Grisar J, et al. Bone turnover markers and sex hormones in men with idiopathic osteoporosis. Eur J Clin Invest 2001;31:444–451
14. Barrett-Connor E, Goodman-Gruen D, Patay B. Endogenous sex hormones and cognitive function in older men. J Clin Endocrinol Metab 1999;84:3681–3685
15. Vermeulen A, Kaufman JM. Aging of the hypothalamo-pituitary-testicular axis in men. Horm Res 1995;43:25–28
16. Morley JE, Perry HM III. Androgen deficiency in aging men. Med Clin North Am 1999;83:1279–1289
17. Nieschlag E, Behre HM. Testosterone: Action, Deficiency, Substitution. 2nd ed. Berlin: Springer; 1998.
18. Morley JE, Kaiser FE, Perry HM, et al. Longitudinal changes in testosterone, luteinizing hormone, and follicular-stimulating hormones in healthy older men. Metabolism 1997;46: 410–413
19. Gray A, Feldman HA, McKinlay JB, et al. Age, disease, and changing sex hormones levels in middle-aged men: results of the Massachusetts Male Aging Study. J Clin Endocrinol Metab 1991;73:1016–1025
20. Winters SJ, Kelley DE, Goodpaster B. The analog free testosterone assay: are the results in men clinically useful? Clin Chem 1998;44:2178–2182
21. Vermeulen A. Decreased androgen levels and obesity in men. Ann Med 1996;28:13–15
22. Marin P, Holmang S, Jonsson L, et al. The effects of testosterone treatment on body composition and metabolism in middle-aged obese men. Int J Obes Relat Metab Disord 1992; 16:991–997
23. Ukkola O, Gagnon J, Rankinen T, et al. Age, body mass index, race and other determinants of steroid hormone variability: the HERITAGE Family Study. Eur J Endocrinol 2001; 145:1–9
24. Morley JE, Charlton E, Patrick P, et al. Validation of screening questionnaire for androgen deficiency in aging males. Metabolism 2000;49:1239–1242
25. Sheikh JI, Yasavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a short version. In: Brink TL, Clinical Gerontology: A Guide to Assessment and Intervention. New York, NY: Haworth Press; 1986
26. Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for clinician. J Psychiatr Res 1975;12:189–198
27. Tan RS, Pu SJ. The andropause and memory loss: is there a link between androgen decline and dementia in the aging male? Asian J Androl 2001;3:169–174
28. Klee GG, Heser DW. Techniques to measure testosterone in the elderly. Mayo Clin Proc 2000;75:S19–S25
29. Fuse H, Kazama T, Ohta S, et al. Relationship between zinc concentrations in seminal plasma and various sperm parameters. Int Urol Nephrol 1999;31:401–408
30. Matsumoto AM. Andropause: clinical implications of the decline in serum testosterone levels with aging in men. J Gerontol A Biol Sci Med Sci 2002;57A:M76–M99
31. Zmuda JM. Exercise increases serum testosterone and SHBG in older men. Metabolism 1996;45:935–939
< div class='tao-gold-member'>









