Patient-Related Risk Factors
Risk factors for CIAKI can be categorized as patient related or procedure related (
Table 33.1). CIAKI rarely develops in the absence of patient-related risk factors, which are collectively characterized by functional and structural changes impairing the capacity of the kidneys to adequately compensate for the hemodynamic and microcirculatory stresses caused by iodinated contrast media. Preexisting disease of the renal parenchyma (i.e., CKD) is characterized by an abnormal medullary microcirculation and a diminished capacity to compensate for hemodynamic perturbation. Clinical studies confirm that underlying renal impairment is the major patient-related risk factor for CIAKI, with increasing levels of dysfunction associated with escalating levels of risk.
50 In a study of 378 hospitalized patients undergoing nonrenal angiography, D’Elia et al.
51 found that preexisting renal insufficiency was the only risk factor predisposing to CIAKI. In an analysis of nearly 3,700 patients, McCullough and colleagues
52 found a strong inverse relationship between the baseline kidney function and a risk of both CIAKI and the need for acute dialysis. Schemata outlining the risk associated with varying levels of CKD have been proposed. For patients with mild to moderate underlying renal insufficiency, the incidence of CIAKI is approximately 5% to 10%. Superimposition of diabetes mellitus on mild to moderate renal insufficiency heightens this risk, whereas the incidence of CIAKI increases to as much as 50% or more in patients with very advanced CKD.
Although diabetes mellitus substantially amplifies the risk of CIAKI in patients with underlying renal impairment, the presence of diabetes in the setting of intact kidney function does not appear to be associated with an increased risk of CKAKI.
51,
52,
53,
54,
55 For example, in a study by Rudnick et al.
53 that compared the effects of high- and low-osmolal contrast agents in 1,196 patients undergoing coronary angiography, CIAKI—defined by a postprocedure increase in serum creatinine (SCr) of ≥ 1.0 mg per deciliter—occurred in none of 359 patients without diabetes or underlying CKD and in just 2 of 315 (0.6%) patients with diabetes but no underlying CKD. However, in study participants with baseline renal impairment, 17 of 296 nondiabetics (6%) and 42 of 213 diabetics (20%) developed CIAKI. Thus, diabetes substantially amplifies the risk of CIAKI in patients with impaired kidney function, but does not appear to represent a notable risk factor in the setting of intact kidney function.
Patients with absolute or effective intravascular volume depletion have increased susceptibility to renal injury from iodinated radiocontrast media.
56 Both clinical states are associated with reduced renal blood flow, which can exacerbate the impact of renal vasoconstriction following intravascular radiocontrast administration. Absolute extracellular volume depletion due to gastrointestinal (GI) losses or diuresis and effective intravascular volume depletion due to advanced heart failure or end-stage liver disease, which are associated with an increased reliance on the vasodilatory effects of prostaglandins to maintain renal microperfusion, also augment the risk for CIAKI.
56,
57 Similarly, nephrotoxic medications, specifically nonselective and cyclo-oxygenase-2 selective nonsteroidal anti-inflammatory medications, which inhibit vasodilatory prostaglandins in the kidney, are associated with an increased risk of CIAKI.
58 Other medications that may also increase the likelihood of contrast-associated renal injury include aminoglycosides and calcineurin inhibitors.
Additional factors that have been reported to increase the risk of CIAKI include older age, hypertension, and anemia.
59,
60 However, the independent impact of these factors on the risk for CIAKI is uncertain because each is strongly correlated with the presence of underlying CKD. Recent studies suggest that an elevated serum glucose concentration at the time of contrast administration may confer an added risk of CIAKI, particularly among nondiabetics.
61 Likewise, elevated urinary protein excretion has been shown to be associated with increased risk of AKI in several clinical settings, although its role in the context of contrast administration is currently unknown.
62 In animal studies, intratubular light chains, particularly if accompanied by intravascular volume depletion, augment the nephrotoxic potential of radiocontrast media.
34 However, more recent studies in humans do not support an association of multiple myeloma with an increased risk for CIAKI.
63 An analysis of 476 patients with multiple myeloma who received iodinated contrast revealed
an incidence of CIAKI of just 0.6% to 1.25%.
64 Early reports of CIAKI following contrast exposure in patients with multiple myeloma may not have fully accounted for other comorbid factors such as sepsis and volume depletion.
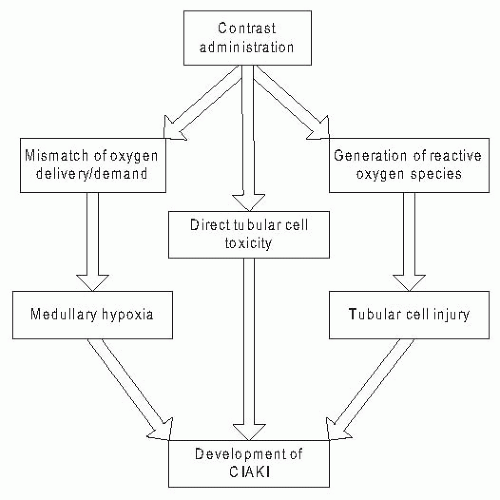
In summary, the administration of iodinated contrast media leads to hemodynamic and toxic effects that, in healthy subjects, are balanced by protective regulatory systems that maintain renal parenchymal oxygenation, function, and integrity. These protective regulatory systems are impaired in the setting of patient-related risk factors, particularly preexisting CKD, leading to an increased susceptibility to renal injury and the development of CIAKI.
Procedure-Related Risk Factors
A series of procedure-related factors have been identified that increase the risk for CIAKI. The dose of contrast has been the subject of considerable attention, with some studies demonstrating an association of higher volumes of contrast with greater risk and other studies showing no such association.
65,
66,
67 Miller and colleagues
67 prospectively evaluated 200 patients undergoing procedures with intravenous or intra-arterial contrast and reported no consistent change in renal function with increasing doses of contrast media. Conversely, Cigarroa et al.
65 demonstrated that decreasing the volume of contrast administered during coronary angiography was associated with a reduction in the incidence of CIAKI. Although a specific threshold volume of contrast above which the risk for CIAKI increases substantially has not been definitively determined, multiple sequential procedures or procedures employing larger volumes of contrast appear to pose a greater risk. Similarly, higher doses of iodine have also been associated with a greater risk for CIAKI, which has led to the development of formulas that incorporate the dose of iodine to estimate risk.
68,
69 However, the role and importance of an iodine dose relative to the overall volume of contrast requires further study.
The type of contrast agent has also been strongly associated with risk for CIAKI. The first iodinated contrast media widely used in clinical practice were high-osmolal ionic derivatives of triiodobenzene, such as diatrizoate, meglumine, or metrizoate.
70 These high-osmolal contrast media (HOCM) were characterized by osmolalities that were five to eight times greater than blood (approximately 1,500 to 2,000 mOsm per kilogram of water). A series of studies in the early 1990s demonstrated that contrast agents with osmolalities of approximately 600 to 850 mOsm per kilogram (low-osmolal contrast media [LOCM]) were less nephrotoxic than conventional HOCM.
53,
71 A third-generation iso-osmolal agent (approximately 300 mOsm per kilogram), iodixanol, was introduced in the late 1990s. Several clinical trials and meta-analyses have compared the nephrotoxicity of this agent with various LOCM, with conflicting results (see section on prevention).
72,
73,
74,
75Iodinated contrast media are administered intra-arterially in the setting of an angiography and intravenously with computed tomography (CT). Direct comparisons of the incidence of CIAKI across different procedure types have been scarce. In a study of 660 patients with CKD, Weisbord et al.
76 demonstrated that the incidence of CIAKI, defined by an increase in SCr ≥25%, was higher following noncoronary angiography (13.2%) than both coronary angiography (8.5%) and CT imaging (6.5%). However, these analyses did not account for variation in baseline clinical risk factors such as diabetes mellitus, heart failure, and the severity of CKD or in differential application of preventive interventions such as IV fluids. Differences in such factors may account for the higher observed rate of CIAKI following angiography in this study and may also underlie the perception that procedures involving intra-arterial contrast administration are associated with a higher risk of CIAKI than procedures that use IV injection.