General Considerations
Continuous renal replacement therapy (CRRT) represents a number of technically distinct modalities characterized by slow per-minute solute clearance and ultrafiltration rates that are spread over most or all of the day to minimize wide metabolic or volume shifts. Continuous techniques were originally described in the 1970s as experimental treatment for diuretic-resistant, hypervolemic patients whose clinical picture made them inappropriate candidates for either peritoneal dialysis (PD) or intermittent hemodialysis (IHD). Since then, the presence of CRRT in the intensive care unit (ICU) has evolved both in scope and complexity, and as a result, CRRT techniques are quickly becoming the standard of care for critically ill patients with acute renal failure (ARF). This chapter will review the major terms used in the delivery of CRRT, the most prominent clinical and technical issues encountered by the healthcare teams, and promising technologies that may improve the efficacy and future applicability of the relevant modalities.
Treatment
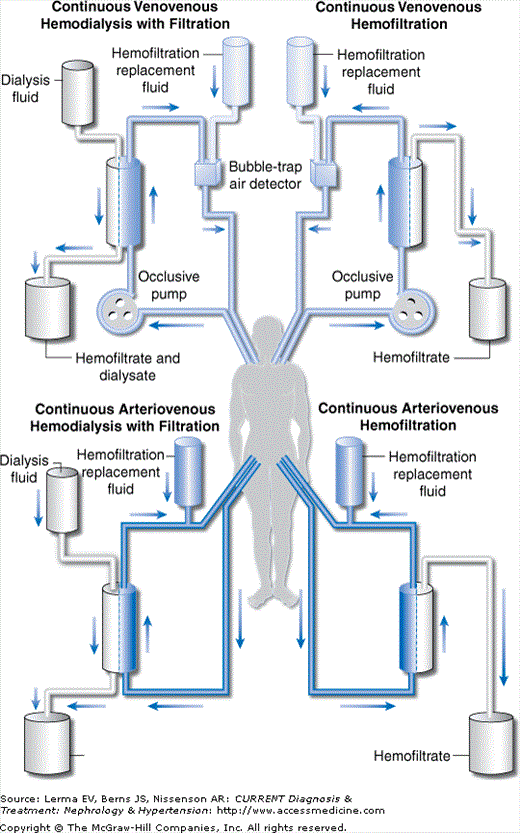
Distinct CRRT techniques are generally defined by the vascular access used and the mechanism of solute/water removal relied upon to maintain the desired clinical parameters (Figure 52–1).
Figure 52–1.
The mechanisms of hemofiltration and hemodialysis with filtration. No attempt has been made to represent the automatic control of the rates of filtration and infusion of replacement fluid. (Reproduced with permission from Forni LG, Hilton PJ: Continuous hemofiltration in the treatment of acute renal failure. N Engl J Med 1997;336:1303.)
In arteriovenous (AV) techniques the pressure gradient for solute and water removal is supplied by the difference in pressures between the patient’s arterial and venous vasculature. These techniques necessitate arterial cannulation with its attendant risks of arterial thrombosis, limb ischemia, hemorrhage, and atheroembolism (among others). Furthermore, the amount of solute and water removal is restricted by each individual patient’s hemodynamic status, making both delivery of adequate therapy and standardization of delivered therapy difficult, especially in hypotensive critically ill patients. The major advantage of AV access is the ability to deliver therapy without complicated external machinery and support. However, because of the associated high risk-to-benefit ratio and lack of control over blood flow rates, AV techniques have fallen out of favor in most tertiary care centers.
In venovenous (VV) techniques the pressure gradient for solute and water removal is supplied by an occlusive peristaltic pump. VV access allows avoidance of arterial cannulation and greater control over and greater reliability of the rate of blood flow into the extracorporeal circuit. Disadvantages of VV access include incorporation of more external devices (ie, pumps/air traps) and longer circuits that are more prone to clotting. Nevertheless, because of the superior safety and control associated with VV access as compared to AV access, VV techniques are used in the majority of clinical settings in which CRRT is indicated.
There are several different modalities for solute and water removal, as described in the following and in Figure 52–2 and Table 52–1. In the current environment, hemofiltration and hemodiafiltration techniques are most commonly used.
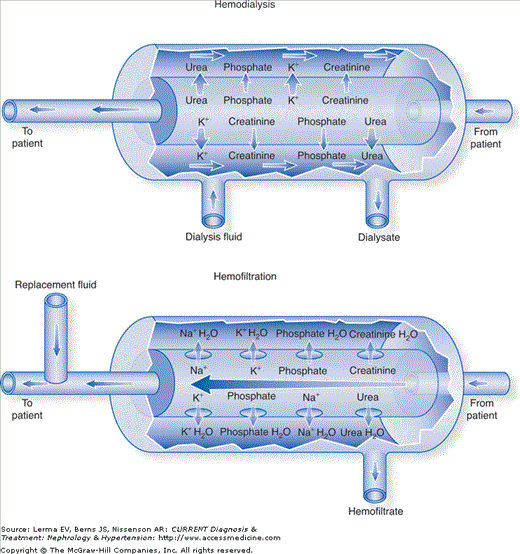
Figure 52–2.
Hemodialysis and hemofiltration. The arrows that cross the membrane indicate the predominant direction of movement of each solute through the membrane; the relative size of the arrows indicates the net amounts of the solute transferred. Other arrows indicate the direction of flow. (Reproduced with permission from Forni LG, Hilton PJ: Continuous hemofiltration in the treatment of acute renal failure. N Engl J Med 1997;336:1303.)
IHD | CVVH | CVVHD | CVVHDF | |
|---|---|---|---|---|
Membrane | Variable permeability | High permeability | Hemodiafilter | Hemodiafilter |
Anticoagulation | Short | Prolonged | Prolonged | Prolonged |
Blood flow rate, mL/minute | >250 | 200–250 | 100–200 | 100–200 |
Dialysate flow rate, mL/minute | >500 | — | 17–34 | 17–34 |
Ultrafiltration, L/hour | 0–2 | 1–2 | <0.2 | 0.7–1.5 |
Dialysate base | Acetate 4, Bicarb 35 m/Eq/L | — | Lactate 35 or Bicarb 35 None (citrate) | Lactate 35 or Bicarb 35 None (citrate) |
Substitution fluid | — | Variable amount and composition | None | Variable amount and composition |
Duration | Hours | Days | Days | Days |
Urea clearance, mL/minute | Diffusion 180–200 | Convection 17–34 | Diffusion 17–34 | Both 17–34 |
In hemodialysis (HD), solute transport is almost exclusively diffusive and generally favors clearance of small molecules less than ˜300 Da in size. The patient’s blood and dialysate are separated by a semipermeable membrane with relatively small pores (ie, low-flux). Electrolytes and other solute particles small enough to pass through membrane pores diffuse freely down their concentration gradients, leading theoretically to equal concentrations on either side of the membrane, assuming their sieving coefficient (the ratio of solute concentration in dialysate to blood) is 1. In intermittent hemodialysis, dialysate flow rates are high (ie, >800 mL/minute) in comparison to blood flow rates, allowing a constant maximal concentration gradient between the two sides of the membrane and a resultant rapid solute transport. In continuous hemodialysis, dialysate (17–34 mL/minute) and blood flow rates are lower (100–200 mL/minute), allowing near or complete equilibration between compartments and a resultant gradual solute transport. Thus, per-minute solute clearance using continuous HD is less than that achievable with IHD. However, because continuous techniques have the luxury of essentially unlimited time, the average daily solute clearance can be equal to or greater than in IHD. Water clearance can also be achieved by hydrostatically forcing plasma water across the membrane. The rate of water removal is usually low, does not result in either hypovolemia or hemoconcentration, and does not generally contribute significantly to solute clearance.
In hemofiltration (H or HF) solute transport is convective and can effectively clear substances up to 20 kDa in size. Plasma water is forced across the filter based on the transmembrane pressure difference (TMP) between the blood and filtrate sides of the filter. Solute particles that are smaller than the filter pores can be “dragged” across into the ultrafiltrate (UF) with plasma water and are in the same concentration in the UF as they are in the prefilter plasma. The magnitude of water and solute clearance is proportional to the amount of UF formed, and can be manipulated by changing the TMP (ie, by increasing the blood flow or by applying suction to the filtrate side). In general, the high rate of UF in this modality would result in hemoconcentration and hypovolemia if left unchecked, so physiologic replacement fluids are usually infused into the circuit either before (predilution) or after (postdilution) the blood interacts with the filter. Thus, over time, the infusion of “clean” replacement fluids essentially “flushes” out the patient’s extracellular water by replacing the “dirty” UF. The composition of the replacement fluid and the infusion rate can also be adjusted to meet specific electrolyte or volume management goals. HF has the theoretical benefit of superior middle-sized molecule clearance in comparison to diffusive therapies.
In hemodiafiltration (HDF), dialysate is run countercurrent to blood flow and a positive TMP from blood to dialysate is created, yielding both diffusive and convective clearance as previously described. The amount of UF created necessitates replacement fluid infusion. HDF represents a marriage of HF and HD and may enjoy the benefits of increased convective middle molecule and diffusive small molecule clearances.
Slow continuous ultrafiltration (SCUF) is similar to HF in that water and solute clearance is convective. However, the amount of ultrafiltrate created is small (generally 2–4 mL/minute) in comparison to HF and does not require replacement fluid infusion. SCUF is generally used when loss of plasma water (and not solute clearance) is the main goal of therapy.
The specific combination of vascular access and water/solute transport modality used allows for standardized acronyms for each of these therapies (eg, continuous venovenous hemodiafiltration = CVVHDF).
Because continuous and intermittent dialysis techniques have different strengths and weaknesses, the goals of therapy and the advantages/disadvantages of each modality must be determined prior to choosing a renal replacement therapy. The proper treatment then can be matched to each patient’s clinical needs.
As a general group, continuous techniques enjoy theoretical advantages over IHD.
Hypotension is one of the most common complications associated with intermittent hemodialysis, occurring in approximately 20–30% of all treatments. In critically ill patients, the majority of whom are already hemodynamically unstable, further iatrogenic hypotensive events may lead to further organ ischemia and injury. Several prospective and retrospective studies have demonstrated better hemodynamic stability associated with CRRT, although this has not been rigorously validated.
Although the clearance rate of small solutes is slower per unit time with CRRT, the continuous nature of the therapy leads to urea clearances that are more efficient after 48 hours than with alternate day intermittent hemodialysis.
In the ICU, nutritional requirements (ie, total parenteral nutrition) and the use of intravenous medications often necessitate the administration of large amounts of fluid. The inability to severely restrict fluid intake in ICU patients can result in excessive volume overload, which may compromise tissue perfusion and has been associated with adverse outcomes. Attempts to restrict fluid in this setting may additionally compromise adequate nutrition. The ability to adjust fluid balance as often as hourly, even in hemodynamically unstable patients, is in large part responsible for the growing popularity of CRRT among intensivists.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree