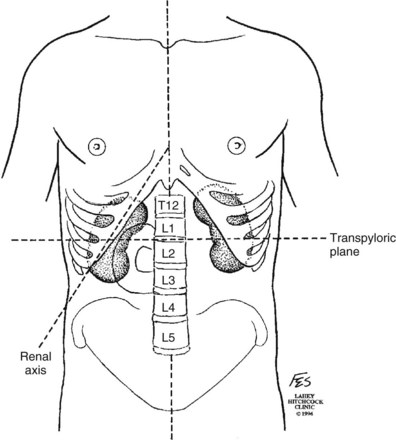
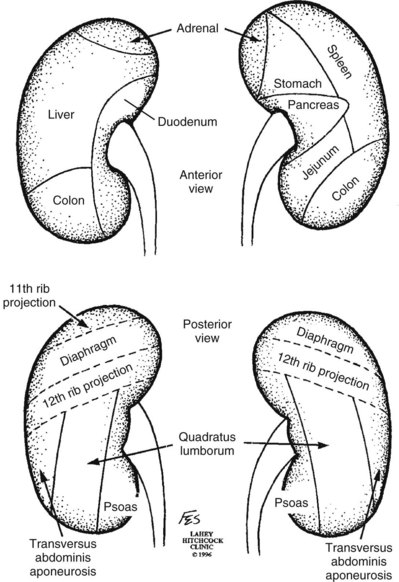
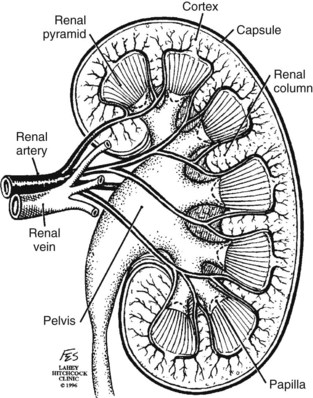
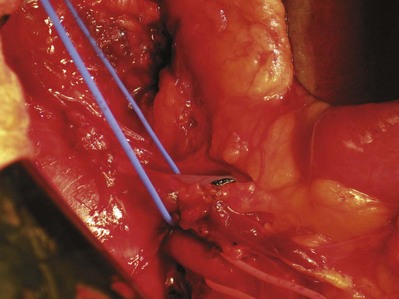
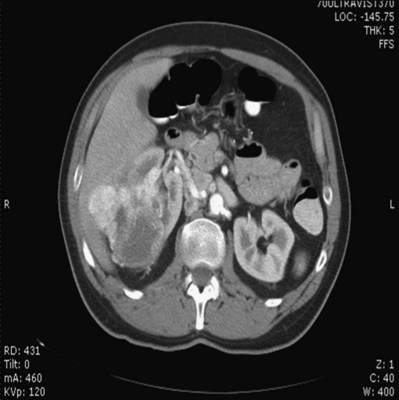
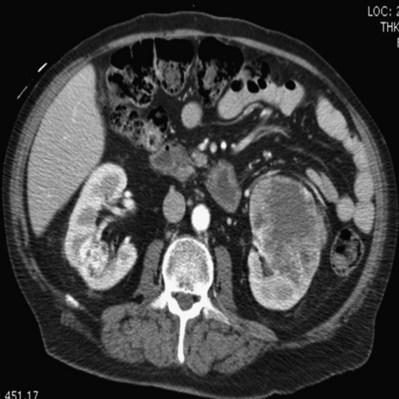
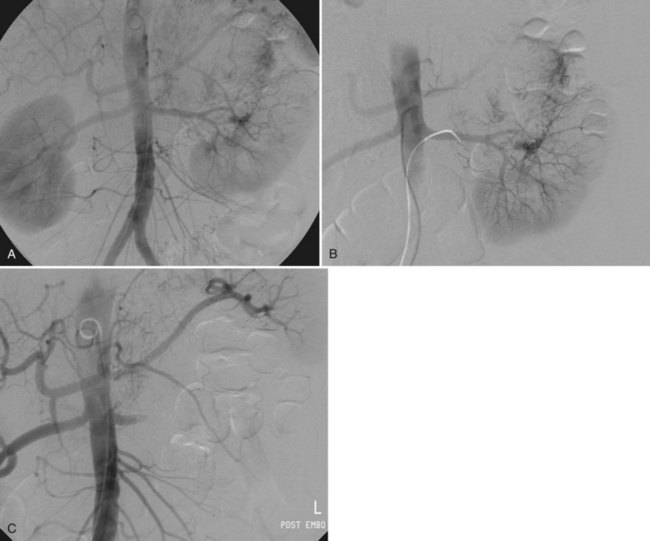
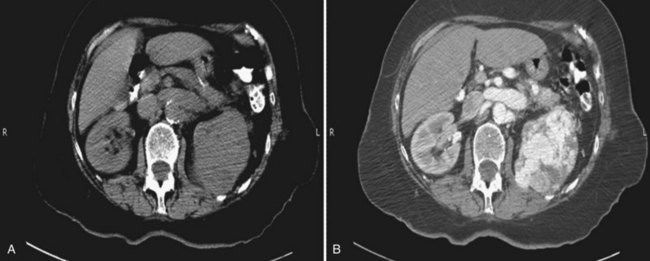
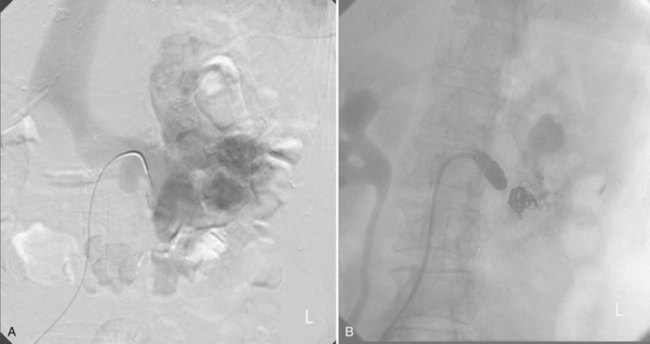
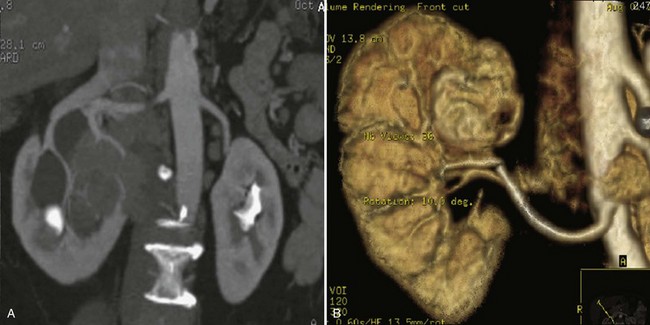
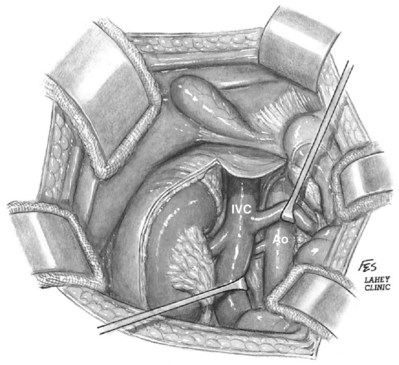
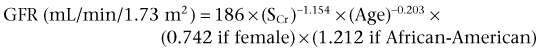Patrick A. Kenney, MD, Chad Wotkowicz, MD, John A. Libertino, MD The history of open renal surgery is characterized by the spirit of innovation, investigation, and excellence that distinguishes modern urology (Table 54–1). Despite progress in minimally invasive techniques and other alternatives, open renal surgery remains a vital field that continues to benefit from refinement and research. Open surgery is an essential component of the contemporary, cutting-edge surgical armamentarium. Table 54–1 Important Advances in Open Renal Surgery Knowledge of regional anatomy is vital to safe and efficient renal surgery (Figs. 54-1 to 54-4 on the Expert Consult website The origin of the right renal artery is beneath the left renal vein and it typically passes posterior to the inferior vena cava (IVC) (Fig. 54–5; see also Fig. 1–33). During transabdominal left radical nephrectomy, knowledge of this anatomic relationship may prevent inadvertent ligation of the right renal artery (Fig. 54–6; see Fig. 54–7 on the Expert Consult website Figure 54–5 Arterial anatomy can be well defined using CT (A) and three-dimensional reconstruction (B). Figure 54–6 Relationship of right kidney to great vessels. IVC, inferior vena cava; Ao, aorta. (© The Lahey Clinic.) The kidney is divided into four segments (superior, anterior, posterior, inferior) that are supplied by one or more segmental renal arteries, which are end arteries (Fig. 54–8 on the Expert Consult website Typically, the first division of the renal artery creates the anterior and posterior segmental branches (see Fig. 1–30A and B). The posterior segmental branch of the main renal artery arises proximal to the hilum, passes posterior to the renal pelvis, and supplies the posterior renal segment. Classically, a crossing vessel responsible for ureteropelvic junction (UPJ) obstruction is an aberrant posterior segmental artery passing anterior to the UPJ. The anterior segmental branch of the main renal artery divides into four divisions: the apical (superior) segmental artery, the anterior superior segmental artery, the anterior inferior segmental artery, and the basilar (inferior) segmental artery. These are also referred to as the apical, upper, middle, and lower anterior segmental arteries, respectively. These segmental arteries then divide further into lobar arteries (associated with each renal pyramid) and further into two or three interlobar arteries (Fig. 54–10). The interlobar arteries travel through the renal medulla alongside either side of the pyramids. They divide at the corticomedullary junction into arcuate arteries that give rise to the interlobular arteries that travel radially to the capsule. They give off numerous side branches en route, including the afferent arterioles. In parallel to the renal arterial system, the renal venous system progresses from the interlobular veins to the arcuate, interlobar, lobar, and segmental veins (see Fig. 1–32). The segmental veins combine to form three to five venous tributaries, the confluence of which forms the main renal vein near the hilum. Each kidney is typically drained by a single renal vein that courses anterior to the renal artery and joins the lateral aspect of the vena cava. The left renal vein is longer than the right, making the left kidney attractive for donor nephrectomy. The left renal vein typically passes anterior to the aorta. Frequent anatomic variation in the renal vasculature contributes to the complexity of open renal surgery. Multiple renal arteries, which typically arise from the aorta or iliac arteries, are the most common variation, occurring in 25% to 30% of the population (Merklin and Michels, 1958; Boijsen, 1959; April, 1997). Supernumerary hilar renal arteries originate at the aorta adjacent to the renal artery and traverse the renal hilum, whereas polar renal arteries originate at a greater distance from the main renal artery and enter the renal parenchyma directly. Ectopic and horseshoe kidneys more frequently have supernumerary renal arteries (Fig. 54–11). Figure 54–11 Horseshoe kidney with multiple arterial branches well defined with three-dimensional imaging. Venous anomalies occur less frequently. Supernumerary renal veins occur less commonly on the left than right (Merklin and Michels, 1958). The most common variation is duplication of a renal vein (6%) (April, 1997). Retroaortic left renal veins occur in approximately 2% of patients. Circumaortic renal veins are present in 1.6% to 6.0% of the population (Kottra and Castellino, 1970). One must be vigilant to identify these infrequent anomalies during preoperative evaluation or intraoperative dissection. Lymphatics parallel the arterial system from the renal cortex into the sinus and then empty into hilar nodes associated with the renal vein (see Figs. 1–34 [on the Expert Consult website Open renal surgery often requires prolonged nonphysiologic positioning with attendant impingement of cardiovascular and pulmonary function. The flank position may impede ventilatory capacity as well as decrease venous return to the heart, with resultant reduction in cardiac output. Patients may have significant bleeding, fluid shifts, or increased oxygen demand, which can result in imbalance in oxygen supply and demand. Preoperative evaluation including laboratory and imaging studies, along with judicious specialist consultation is essential (Table 54–2). Table 54–2 Preoperative Workup: Basic Components * If deemed necessary after assessment of cardiac and pulmonary risk factors. Some patients will benefit from referral to a consulting cardiologist for consideration of resting 12-lead electrocardiogram, noninvasive evaluation of left ventricular function, noninvasive stress testing, medication adjustments, or preoperative coronary revascularization (coronary artery bypass graft or percutaneous coronary intervention). Patients with unstable coronary syndromes, decompensated heart failure, significant arrhythmias, or severe valvular disease should be evaluated and treated by a cardiologist before noncardiac surgery (Fleisher et al, 2007). It is incumbent on the urologist to identify these patients and others who are at risk of cardiovascular complications and to refer them for evaluation by a specialist. In addition, patients with known coronary artery disease or signs or symptoms suggestive of new coronary artery disease should receive a preoperative cardiac assessment (Fleisher et al, 2007). In asymptomatic patients, referral should be made based on a history and physical examination (Lee et al, 1999; Fleisher et al, 2007). The history should elucidate symptoms of cardiovascular disease, risk factors for cardiac disease, and pertinent medications (Table 54–3). The Revised Cardiac Risk Index (RCRI), a simple validated index to predict cardiovascular complications, was developed at a tertiary care facility for prediction of cardiac risk of major noncardiac surgery in patients 50 years of age or older (Lee et al, 1999). The RCRI, which is composed of six independent predictors of cardiac complications, identifies patients at risk of cardiac complications (Table 54–4) and may be helpful for preoperative risk stratification. Table 54–3 Pertinent Cardiovascular History Table 54–4 Revised Cardiac Risk Index From Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999;100(10):1043–9. In noncardiothoracic surgery, risk factors for postoperative pulmonary complications include chronic obstructive pulmonary disease, age older than 60 years, inhaled tobacco use, American Society of Anesthesiologists (ASA) class II or higher, functional dependence (i.e., inability to perform activities of daily living or need for equipment and assistance for some activities of daily living), congestive heart failure, pulmonary hypertension, and hypoalbuminemia (Qaseem et al, 2006; Bapoje et al, 2007). The urologist should screen for these risk factors and refer the patient for preoperative pulmonary evaluation and risk reduction. Even in the absence of baseline risk factors, open renal surgery itself may disturb a patient’s pulmonary equilibrium and render him or her at risk for postoperative pulmonary complications. For instance, transection of upper abdominal and flank muscles, removal of ribs, impairment of diaphragm function through nerve or muscular injury, pneumothorax, hemothorax, and pleuritic pain may all contribute to pulmonary difficulties. Prolonged surgery (>3 hours) is an independent predictor of pulmonary complications, as is either thoracic or upper abdominal surgery (Qaseem et al, 2006). Pulmonology consultation, preoperative pulmonary function testing, chest radiography, or arterial blood gas analysis may benefit patients who are at risk of pulmonary complications. Preoperative interventions such as smoking cessation 6 to 8 weeks before surgery and inspiratory muscle training may reduce the risk of pulmonary complications (Bapoje et al, 2007). In patients with significant pulmonary risk factors or impairment an anterior surgical approach in the supine position might be preferable to the flank approach (Novick, 2007). Risk can be ameliorated after surgery by deep breathing exercises or incentive spirometry and by selective rather than routine use of nasogastric drainage (Qaseem et al, 2006; Bapoje et al, 2007). Initial renal evaluation should include urinalysis and measurement of serum creatinine concentration. The serum creatinine value is the most widely used surrogate marker of glomerular filtration rate (GFR) but is not ideal. Changes in the serum creatinine level can reflect a change in GFR but may also represent a change in production of creatinine or tubular secretion of creatinine. A better estimate of GFR is provided by 24-hour creatinine clearance (CrCl), which reflects the volume of plasma that is cleared of creatinine per minute. Because creatinine is also cleared by tubular secretion in addition to filtration, the 24-hour CrCl tends to overestimate GFR. GFR can also be estimated by renal radionuclide imaging. For instance, renal scan with technetium-99 diethylenetriaminepentaacetic acid (DTPA) can provide an estimate of GFR but its use is limited in patients with renal insufficiency. A commonly used method, which is favored by the senior author, is to estimate GFR using the Modification of Diet in Renal Disease (MDRD) Study equation (Levey et al, 1999): Three-dimensional (3D) volume-rendered CT is a valuable tool in the evaluation of patients undergoing renal surgery (Coll et al, 1999; Derweesh et al, 2003). CT can demonstrate vital information including the anatomy of the renal vasculature, orientation of the kidney, and characteristics of renal tumors, including location, depth of penetration into the kidney, relationship with collecting system, and segmental arterial supply to the tumor (Coll et al, 1999) (Figs. 54-12 to 54-14). CT is also able to characterize other surgically relevant processes, including renal arterial disease, nephrolithiasis, and hydroureteronephrosis (Herts, 2005). The importance of imaging to open renal surgery is likely to increase with time. Future possibilities include image-guided open surgery, the objective of which is to provide surgeons enhanced visualization of the surgical field by merging data from preoperative images with an intraoperative view (Benincasa et al, 2008). In addition, simulation of complex nephron-sparing surgery based on 3D imaging may be possible (Wunderlich et al, 2000). Since its introduction in the early 1970s, renal artery embolization (RAE) has been employed for palliation of inoperable renal tumors, to control bleeding, and as part of multimodal treatment of hypervascular metastatic disease (Almgard et al, 1973). In addition, RAE is used as a preoperative adjunct to resection of locally advanced renal tumors, with or without metastases (Paster et al, 1975) (Fig. 54–15). Proposed benefits of preoperative RAE include shrinkage of an arterialized tumor thrombus to ease surgical removal, reduced blood loss, facilitation of dissection due to tissue plane edema, ability to ligate the renal vein before the renal artery at time of nephrectomy, and modulation of the immune response (Klimberg et al, 1985; Bakal et al, 1993; Kalman and Varenhorst, 1999; Schwartz et al, 2007; Wotkowicz and Libertino, 2007; Wszolek et al, 2008). Ligation of the renal vein before the renal artery is useful in the setting of hilar tumors, tumors with significant medial extension, or considerable perihilar adenopathy (Schwartz et al, 2007; Wotkowicz and Libertino, 2007; Wszolek et al, 2008). Before ligating the renal vein, one should characterize the completeness of embolization to prevent unnecessary blood loss in cases of incomplete embolization. In the senior author’s experience this assessment can be made by evaluating renal venous return during surgery. In addition, angioembolization can also be useful for renal tumors associated with large arteriovenous malformations that are having a deleterious hemodynamic impact (Figs. 54-16 and 54-17). There may be a survival benefit to preoperative RAE. In a case-control study, preoperative RAE and nephrectomy was associated with a survival benefit when compared with nephrectomy alone (Zielinski et al, 2000). In comparison to matched controls, preoperative RAE was associated with improved overall survival at 5 years (62% vs. 35%, P = .01) and 10 years (47% vs. 23%, P = .01). This possible survival benefit has not been demonstrated in a prospective trial. Although there is no conclusive evidence that RAE has any immunotherapeutic benefit it is plausible that angioinfarction may augment the immune response to the renal tumor (Kalman and Varenhorst, 1999). There are reports of regression of metastases from renal cell carcinoma after RAE and nephrectomy (Mohr and Whitesel, 1979; Swanson et al, 1983). The postinfarction syndrome that is nearly universal may be cytokine mediated. Several studies have shown that RAE is immunomodulatory, with documented changes in natural killer cell activity (Bakke et al, 1982), increased cell-mediated cytotoxicity (Johnson and Kalland, 1984), and alteration in lymphocyte proliferation (Nakano et al, 1983). There are no modern molecular data to support these findings. The postinfarction syndrome, which is characterized by flank pain, nausea, and fever, occurs in approximately three fourths of patients and is the most frequently cited complication of RAE (Schwartz et al, 2007). Other complications, including incomplete embolization, coil migration, and groin hematoma, occurred in fewer than 5% of patients (Schwartz et al, 2007). Rarely cited complications include hyponatremia (Huang et al, 2003), paraplegia due to occlusion of spinal arteries, or inadvertent angioinfarction of other organs such as the colon (Roy et al, 1999). The data regarding preoperative RAE are limited, without any randomized trials evaluating the technique. As a result, in some institutions, surgeons rarely employ preoperative RAE (Boorjian et al, 2007). It is the practice of the senior author to perform preoperative RAE for large renal tumors with hypervascular characteristics or IVC tumor thrombus (Wotkowicz and Libertino, 2007). The ideal timing of nephrectomy after embolization is unclear (Craven et al, 1991; Weckermann et al, 1992; Kalman and Varenhorst, 1999; Schwartz et al, 2007). At the Lahey Clinic, surgery usually is timed 4 weeks after angioinfarction. An imaging study to assess the cranial limit of tumor thrombus is repeated shortly before the operation. Although prospective trials are needed to evaluate the role of preoperative RAE in the treatment of renal cell carcinoma, the senior author has found it a useful preoperative adjunct in the management of locally advanced RCC. A preoperative urinalysis and culture should be obtained for all patients undergoing open renal surgery. Patients with urinary tract infection or bacteriuria should be treated with culture-guided antibiotics before surgery, with the goal of sterilizing the urine or reducing the bacterial count if the urine cannot be sterilized (Wolf et al, 2008). In the absence of active infection or bacteriuria, judicious antimicrobial prophylaxis may reduce the risk of surgical site infection and systemic sepsis. In cases in which the urinary tract is not entered, such as radical nephrectomy, antibiotics should be targeted to skin flora (e.g., a first-generation cephalosporin or clindamycin). The American Urological Association (AUA) advises that in these clean cases the use of antibiotics should be limited to patients with risk factors (Table 54–5) (Wolf et al, 2008). In renal surgery in which the urinary tract is entered both skin and genitourinary flora should be covered (with a first- or second-generation cephalosporin or aminoglycoside with metronidazole or clindamycin, or ampicillin/sulbactam, or fluoroquinolone) (Wolf et al, 2008). Table 54–5 Risk Factors for Surgical Site Infection From Wolf JS Jr, Bennett CJ, Dmochowski RR, et al. Best practice policy statement on urologic surgery antimicrobial prophylaxis. J Urol 2008;179(4):1379–90. Open renal surgery is often complex and time consuming. Antibiotics should be readministered during surgery after one to two half-lives to ensure adequate drug levels are maintained until wound closure (Bratzler and Houck, 2005). Antibiotic prophylaxis should not continue beyond 24 hours (Bratzler and Houck, 2005). In addition to antibiotics, sterile technique is employed to reduce the rate of surgical site infection. Although there is no conclusive evidence to support hair removal as a method of reducing surgical site infection, the authors recommend clipping hair at the incision site before sterile preparation of the field (Tanner et al, 2006). It is the practice of the senior author to use povidone-iodine scrub and paint, although one study has found paint alone to be equivalent to scrub and paint for abdominal surgery (Ellenhorn et al, 2005). One should avoid alcohol-containing products because of flammability (Armstrong et al, 2001). Finally, proper surgical technique with regard to tissue handling, hemostasis, avoidance of unnecessary hypothermia, and excision of devitalized tissues is an essential component of infection prevention (Mangram et al, 1999). There is a paucity of date to guide prophylaxis for venous thromboembolism (VTE) in open renal surgery. Although both the AUA and American College of Chest Physicians (ACCP) guidelines recommend pharmacologic VTE prophylaxis in open renal surgery, the data supporting these recommendations are imperfect (Geerts et al, 2008; Forrest et al, 2009). The ACCP recommends routine VTE prophylaxis in all open urologic procedures, although this recommendation is largely based on data from other surgical specialties and radical retropubic prostatectomy. The ACCP recommends preoperative use of graduated compression stockings along with intermittent pneumatic compression devices, pharmacologic prophylaxis after surgery (low-dose unfractionated heparin, low-molecular-weight heparin, or fondaparinux), or both. In patients at high risk of bleeding, the ACCP advises pharmacologic therapy once the bleeding risk has diminished. The AUA recommends use of mechanical prophylaxis in all patients undergoing open surgery and consideration of pharmacologic prophylaxis in patients with elevated risk for VTE (Tables 54-6 and 54-7). Table 54–6 Venous Thromboembolism: Risk Stratification Data from Geerts and colleagues (2008) and Forrest and colleagues (2009). Table 54–7 Venous Thromboembolism: Risk Factors Data from Geerts and colleagues (2008) and Forrest and colleagues (2009). It is the authors’ practice to apply intermittent pneumatic compression devices before open renal surgery for all patients. In the opinion of the senior author there is not sufficient evidence to support routine use of pharmacologic VTE prophylaxis given the associated risk of hemorrhage. At the Lahey Clinic addition of pharmacologic prophylaxis is determined on a case-by-case basis by subjectively assessing an individual’s risk of VTE (see Tables 54-6 and 54-7). In partial nephrectomy, a procedure with significant risk of hemorrhage, pharmacologic prophylaxis is avoided in the vast majority of cases at the Lahey Clinic. This practice is supported by a retrospective review from Memorial Sloan-Kettering Cancer Center that describes a low prevalence (1.5%) of clinically apparent VTE after radical or partial nephrectomy (Pettus et al, 2006). This is an order of magnitude lower than the estimated 15% to 40% rate of asymptomatic VTE (i.e., found on screening) on which the current ACCP recommendations are based (Geerts et al, 2008). The clinical significance of asymptomatic VTE is unclear. Key Points: Preoperative Evaluation and Preparation Safe, effective open renal surgery is predicated on good functional exposure of the kidney. The kidney is a retroperitoneal structure in close proximity to the great vessels, pancreas, spleen, liver, colon, and duodenum. These neighbors serve as landmarks to guide dissection, as well as potential sources of complications and impediment to adequate exposure. An incision that is too small or inappropriately placed will increase the difficulty of open renal surgery and may adversely impact patient outcomes. Good functional exposure permits the surgeon to perform the planned procedure, diminishes the risk of injury to surrounding structures, and permits definitive treatment of an intraoperative complication, such as injury to a great vessel (Libertino, 1998). Proper exposure limits the amount of required retraction, which can be a source of injury to surrounding structures such as the liver or spleen (Cooper et al, 1996). One must balance numerous variables when selecting a surgical approach to the kidney. The ideal surgical approach is one that is tailored not only to the operation being performed but also to the anatomy as defined on preoperative imaging, previous surgical history, body habitus, and presence of limiting factors such as kyphoscoliosis or pulmonary disease (Libertino, 1998). The primary approaches to the kidney are the subcostal, supracostal, and transcostal flank incisions, the thoracoabdominal approach, the dorsal lumbotomy, as well as the midline, paramedian, and subcostal anterior approaches (Table 54–8). The flank approaches are undertaken with the patient in lateral decubitus position with flexion of the table. Flexion tightens the skin and muscles of the flank and improves exposure by increasing the distance between the iliac crest and costal margin. Positioning for the flank approaches can impede pulmonary function and venous return to the heart, with resultant deficits in ventilation and cardiac output (Longnecker, 2007). When the table is flexed with the kidney bar elevated, ischemic injury to a previously operated on contralateral kidney may result (Matin and Novick, 2001). Flank approaches may not be ideal in patients with preexisting cardiopulmonary deficits or kyphoscoliosis. Each of the flank approaches can result in injury to the intercostal nerves with denervation and paresis of the flank musculature, leading to chronic postoperative pain or flank bulge in 3% to 49% of patients (Ward et al, 1974; Chatterjee et al, 2004). Gravity will tend to move the abdominal panniculus of an obese patient away from the operative field, a distinct advantage of the flank approaches. It is imperative to understand the anatomy of the flank before employing these approaches (see Figs. 1–3, 1–4, 1–5, and 1–8). There are three principal flank approaches: subcostal, supracostal, and through the bed of a resected rib. This approach offers good exposure of the renal parenchyma and upper ureter. It is an advantageous approach for surgery on the lower renal pole, UPJ, and upper ureter (e.g., ureterocalicostomy, pyeloplasty, and stone surgery). Simple nephrectomy, insertion of a nephrostomy tube, and drainage of a perinephric abscess may all be accomplished through a subcostal flank incision. Because the approach is extraperitoneal, contamination of the peritoneal cavity is avoided. Access to the renal hilum and vascular pedicle is poor, which is the most salient disadvantage of this approach. The subcostal flank approach is not appropriate for radical or partial nephrectomy (Libertino, 1998). The incision may be caudal to the kidney, hampering access to the upper pole, renal pelvis, and hilum (Novick, 2007). Exposure may be further hindered by the iliac crest and subcostal nerve, the anterior division of the 12th thoracic nerve. After induction of anesthesia, insertion of an endotracheal tube, and introduction of a Foley catheter into the urinary bladder to monitor urine output, the patient is placed in the lateral position. The head is supported to maintain proper alignment of the cervical spine. The tip of the 12th rib should be positioned over the kidney bar (Fig. 54–18). The patient’s back should be nearly flush with the edge of the table to ensure unfettered access by the surgeon. To preserve stability and prevent forward roll, the dependent leg is flexed at the hip and knee and the top leg is kept straight. A pillow is placed between the knees. An axillary roll is deployed just caudal to the axilla to prevent compression or injury of the axillary neurovascular bundle. Other pressure points, including the upper foot, are padded with foam. The nondependent arm should be placed on a padded Mayo stand so that the arm is horizontal with slight forward rotation at the shoulder. The bed is flexed until the flank muscles are under stretch. A kidney bar can be employed if necessary. The bed is placed in Trendelenburg position so that the flank is rendered parallel to the floor. The patient is secured to the mobile part of operating table with 2-inch wide adhesive tape, which fixes the patient in place while allowing adjustment of flexion. The incision is carried sharply through the subcutaneous tissue, exposing the fascia of the latissimus dorsi and external oblique muscles (Fig. 54–19). Electrocautery is used to incise the muscles in the line of the incision (Fig. 54–20), starting with the latissimus dorsi posteriorly. The serratus posterior inferior muscles, which insert into the lower four ribs, are also encountered in the posterior portion of the wound and transected. In the anterior aspect of the wound the external oblique muscle is divided. These maneuvers expose the fused lumbodorsal fascia, which gives rise to the internal oblique and transversus abdominis muscles. The lumbodorsal fascia and internal oblique muscle are divided (Fig. 54–21). By using two fingers inserted into an opening created in the lumbodorsal fascia at the tip of the 12th rib, the peritoneum is swept medially as the transversus abdominis is split digitally. The subcostal nerve should be identified between the internal oblique and transversus abdominis muscles and spared (Figs. 54-22 and 54-23). Figure 54–19 Superficial incision through flank. (From Libertino JA. Reconstructive urologic surgery. 3rd ed. Philadelphia: Mosby; 1997.) Figure 54–21 Dissection through flank muscles. (From Libertino JA. Reconstructive urologic surgery. 3rd ed. Philadelphia: Mosby; 1997.) Figure 54–22 Opening lumbodorsal fascia to gain entrance to retroperitoneum. (From Libertino JA. Reconstructive urologic surgery. 3rd ed. Philadelphia: Mosby; 1997.)
History
YEAR
INNOVATION
1869
Gustav Simon performed the first nephrectomy in a human to treat a ureterovaginal fistula, after first demonstrating that dogs are able to survive with a solitary kidney (Simon, 1870).
1878
Kocher and Langham use a midline incision to accomplish an anterior transperitoneal nephrectomy (Kocher and Langham, 1878).
1881
Morris performed the first nephrolithotomy (Morris, 1881).
1884
Wells performed an inadvertent partial nephrectomy while removing a perirenal fibrolipoma (Wells, 1884).
1887
Czerny carried out the first planned partial nephrectomy to treat a renal tumor (Herr, 2008).
1891
Kuster completed the first successful dismembered pyeloplasty (Kuster, 1896–1902).
1903
Grégoire performed the first procedure resembling a radical nephrectomy, described as an en-bloc resection of a kidney, perirenal fat, adrenal gland, and lymphatic tissue for cancer (Grégoire, 1903).
1913
Berg employed a transverse abdominal incision and mobilization of the colon to safely control the renal pedicle. He also obtained control of the renal veins and made a cavotomy to extirpate caval tumor thrombi (Berg, 1913).
1922
Rehn performed caval resection with reimplantation of the contralateral renal vein (Rehn, 1922).
1932
Rosenstein performed a palliative partial nephrectomy for renal cancer in a patient with contralateral renal insufficiency, defining an indication for nephron-sparing surgery (Rosenstein, 1921).
1959, 1960
Kerr and Klotz described renal hypothermia to minimize ischemic damage while prolonging available operative time for partial nephrectomy (Kerr et al, 1960; Herr, 2008).
1973
Libertino and Zinman described revascularization of the totally occluded renal artery (Zinman and Libertino, 1973).
1998
Libertino described use of a minimally invasive approach for cardiopulmonary bypass for vena cava thrombectomy (Fitzgerald et al, 1998).
Surgical Anatomy
General
![]() ). The renal capsule, perinephric fat, Gerota fascia (renal fascia), and paranephric fat are important landmarks in staging and surgery of renal malignancy (see Fig. 1–27).
). The renal capsule, perinephric fat, Gerota fascia (renal fascia), and paranephric fat are important landmarks in staging and surgery of renal malignancy (see Fig. 1–27).
Renal Vasculature
Arterial
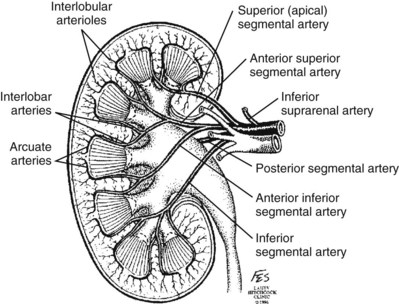
![]() ). The inferior adrenal artery and a branch that supplies the renal pelvis and ureter arise from the renal artery. In the majority of cases the renal artery branches in the distal third of the vessel or the renal sinus.
). The inferior adrenal artery and a branch that supplies the renal pelvis and ureter arise from the renal artery. In the majority of cases the renal artery branches in the distal third of the vessel or the renal sinus.
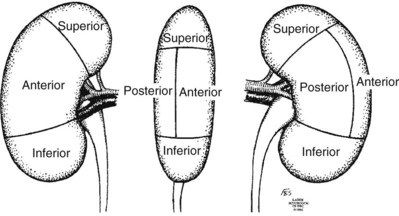
![]() ). Ligation or occlusion of a segmental artery, or any of its branches, leads to devitalization of renal tissue. Intraoperatively, one can administer methylene blue dye and occlude the segmental arteries to define the borders of the vascular segments. Despite variation in the origin of the branches supplying the renal segments, the anatomic pattern of the segments is constant (Graves, 1954). Three planes between the vascular segments can be demonstrated (see Fig. 54–8 on the Expert Consult website
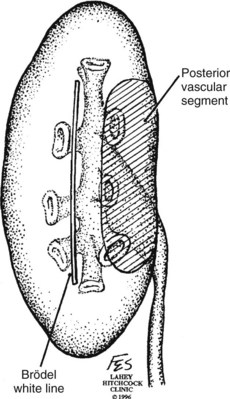
). Ligation or occlusion of a segmental artery, or any of its branches, leads to devitalization of renal tissue. Intraoperatively, one can administer methylene blue dye and occlude the segmental arteries to define the borders of the vascular segments. Despite variation in the origin of the branches supplying the renal segments, the anatomic pattern of the segments is constant (Graves, 1954). Three planes between the vascular segments can be demonstrated (see Fig. 54–8 on the Expert Consult website![]() ) and are important for intrarenal surgery. In theory, incision in the plane between the anterior and posterior segments affords reduced blood loss (Brodel, 1900) and has been described for anatrophic nephrolithotomy (Smith and Boyce, 1968). This plane, located on the posterior surface of the kidney, is not to be confused with the “Brodel white line,” a whitish linear depression on the lateral aspect of the anterior surface of some kidneys formed by fusion of anterior and posterior columns of Bertin, which demarcates a highly vascular area (Brodel, 1900) (Fig. 54–9).
) and are important for intrarenal surgery. In theory, incision in the plane between the anterior and posterior segments affords reduced blood loss (Brodel, 1900) and has been described for anatrophic nephrolithotomy (Smith and Boyce, 1968). This plane, located on the posterior surface of the kidney, is not to be confused with the “Brodel white line,” a whitish linear depression on the lateral aspect of the anterior surface of some kidneys formed by fusion of anterior and posterior columns of Bertin, which demarcates a highly vascular area (Brodel, 1900) (Fig. 54–9).
Venous
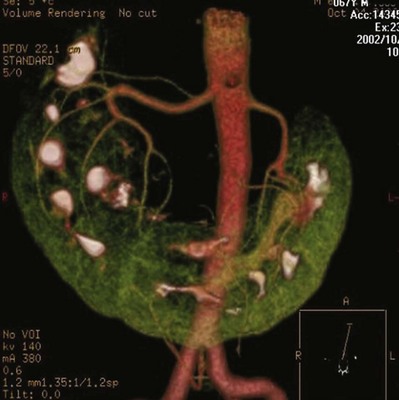
Anomalies of Renal Vasculature
Lymphatics
![]() ] and 1-35). There is communication between parenchymal lymphatics and those of the renal capsule and perinephric fat. From the first description of renal lymphatic drainage in 1935 to modern day lymphatic dissections the complexity of renal lymphatic drainage beyond the hilum has been apparent (Parker, 1935; Assouad et al, 2006). For the surgeon, the most important characteristic of renal lymphatics is variability in drainage. More so than in malignancies of the penis, testis, bladder, and prostate, the role of regional lymphadenectomy in kidney cancer remains controversial.
] and 1-35). There is communication between parenchymal lymphatics and those of the renal capsule and perinephric fat. From the first description of renal lymphatic drainage in 1935 to modern day lymphatic dissections the complexity of renal lymphatic drainage beyond the hilum has been apparent (Parker, 1935; Assouad et al, 2006). For the surgeon, the most important characteristic of renal lymphatics is variability in drainage. More so than in malignancies of the penis, testis, bladder, and prostate, the role of regional lymphadenectomy in kidney cancer remains controversial.
Preoperative Evaluation and Preparation
Identification and Optimization of Cardiovascular and Pulmonary Comorbidities
Cardiovascular
Independent Predictors
NO. OF RISK FACTORS
RATE OF MAJOR CARDIAC COMPLICATION
0
0.4%
1
0.9%
2
7%
3 or more
11%
Pulmonary
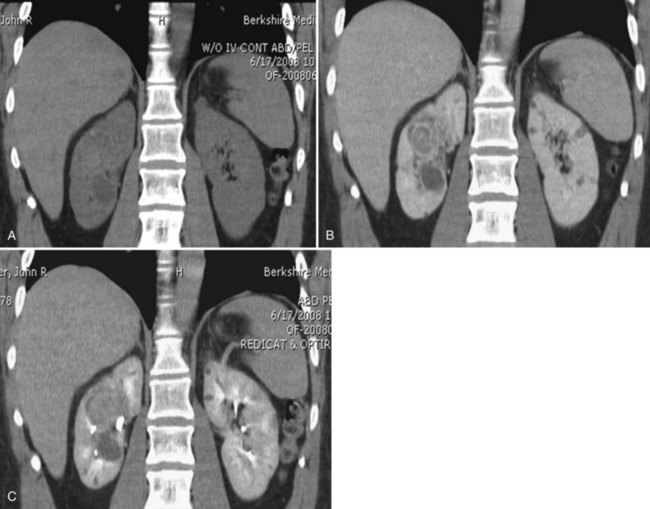
Renal Evaluation
Imaging
Angioembolization
Surgical Site Infection Prophylaxis
Alteration in Host Defense
Alteration in Pathogen
Venous Thromboembolism Prophylaxis
Low Risk
Minor surgery in patients younger than age 40 years with no additional risk factors
Moderate Risk
High Risk
Highest Risk
Surgery in patients with multiple risk factors
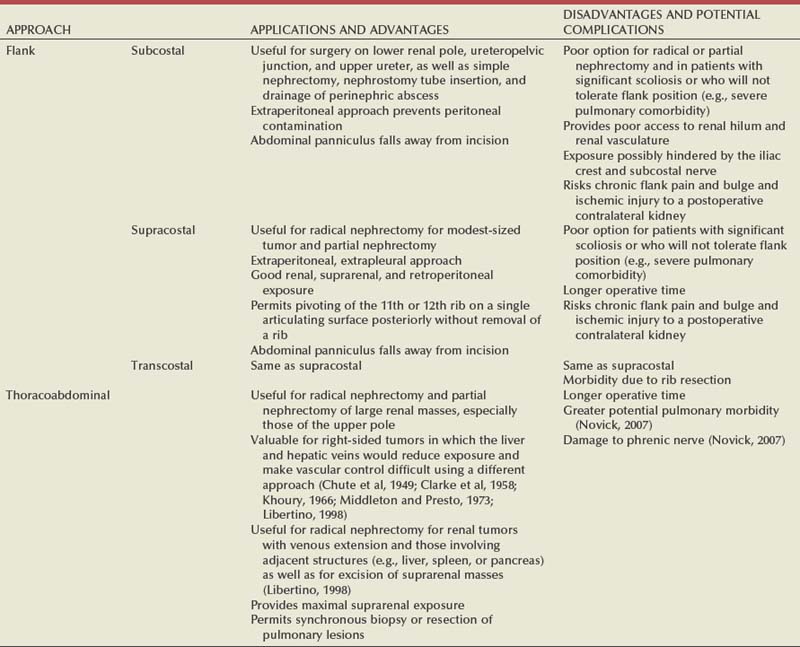
Surgical Approaches
Flank Approaches
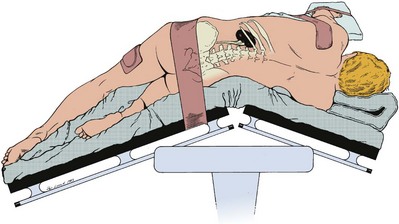
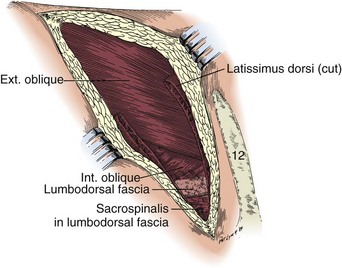
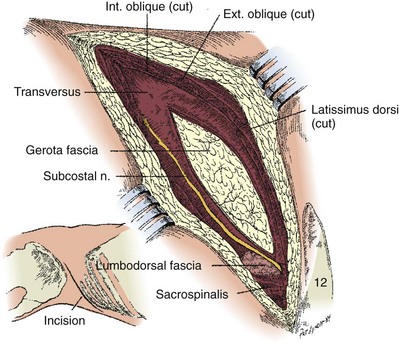
Subcostal Flank Approach
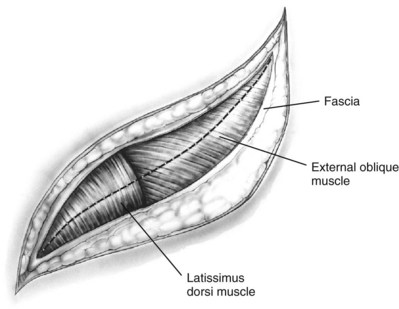
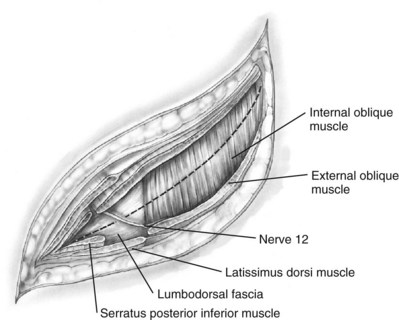
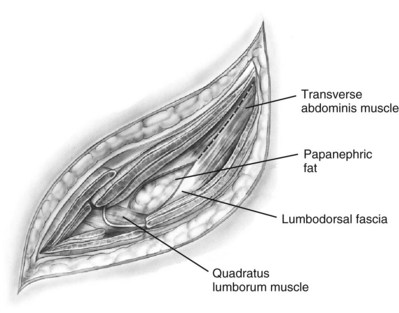
Contemporary Open Surgery of the Kidney
• Patients with unstable coronary syndromes, decompensated heart failure, significant arrhythmias, or severe valvular disease should be evaluated and treated by a cardiologist before noncardiac surgery.