Conservative Therapy for Stress Incontinence
Laura Scheufele
Karen Abraham
INTRODUCTION
Stress urinary incontinence (SUI) is “the complaint of involuntary leakage on effort or exertion, or on sneezing or coughing,” as defined by the International Continence Society (1). Urine leakage from the urethra occurs when the intra-abdominal pressure exceeds the urethral pressure. The reason for the inadequate urethral closure pressure may be multifactorial. Pressure generation is affected by the ability to provide active support (i.e., ability of the smooth and striated muscles of the intrinsic and extrinsic sphincters to generate pressure) and passive support (i.e., nonneuromuscular factors such as the integrity of the connective tissue, vascular plexus, and the urethral lining) to the lower urinary tract. It is the interplay between factors that determines continence.
Interventions for SUI are directed toward improving urethral pressure generation and/or minimizing intra-abdominal pressure. Because of the multifactorial origin of SUI, the best outcomes are often achieved through a combination of interventions. The focus of this chapter is on conservative therapy techniques for SUI. These are nonsurgical techniques that attempt to restore the normal anatomic and mechanical relationships of the lower urinary tract, including pelvic floor muscle exercise, pessary use, lifestyle modifications, and pharmacologic interventions. The Agency for Health Care Policy and Research’s Clinical Practice Guidelines on Urinary Incontinence in Adults advise that “the least invasive and least dangerous procedure that is appropriate for the patient should be the first choice” when treating urinary incontinence (2).
Behavioral management is the treatment of choice for SUI because of the low risk, minimal complications, and noninvasive nature of the techniques. The increased risk and cost of surgical repair make optimizing all nonsurgical therapy a priority. Conservative management should ideally eliminate symptoms and result in long-term improvement in a patient’s quality of life. Although incontinence may not be cured, the severity may be lessened such that incontinence no longer has a significant impact upon the patient’s lifestyle.
Women with SUI often present with impaired ability to provide both active and passive support to the lower urinary tract. The key component of active support is adequate pelvic floor muscle (PFM) function. The active contraction of the intrinsic and extrinsic sphincters and PFM provide a force that closes the bladder outlet (3). If sudden external pressure is exerted on the bladder, as in coughing, the PFMs respond by quickly contracting to prevent leakage (guarding reflex). An active contraction has been noted to occur 250 msec before the increase in intra-abdominal pressure in asymptomatic individuals (4). This contraction actively closes the bladder outlet, countering the abdominal pressure. Women with SUI have been observed to have significantly lower peak contractions, decreased length of maximal PFM contractions, and a progressive decline by decade in maximal PFM electromyographic activity as compared to continent women (5, 6, 7). If PFM function is compromised in any way, such as weakness or impaired
innervation, the guarding reflex will be inadequate, resulting in urine leakage.
innervation, the guarding reflex will be inadequate, resulting in urine leakage.
Women with SUI also have been noted to have impaired passive support of the lower urinary tract. The urothelium and the submucosal vessels of the urethra and bladder neck help to create a “leakproof” mucosal seal (7,8). A urethra that has lost its elasticity and whose submucosal blood supply has been compromised by prior surgery, radiation, obstetric injury, or loss of estrogen may require significantly more force from the active sphincteric mechanism to obtain a urine-tight closure. A hypermobile urethra (i.e., descends with abdominal pressure) allows leakage of urine (9). The slow twitch muscle fibers of the PFMs provide passive support to the urethra and bladder neck at rest, whereas the fast twitch muscle fibers are a part of the active support mechanism (3).
Proper anatomic support of the urethra and its junction with the bladder at the bladder neck is also necessary to resist sudden increases in abdominal pressure. Pressure is transmitted equally to a well-supported urethra and bladder and, therefore, conveys equal force to structures trying to release and to hold urine (3). Tension in the ligaments and fascia supporting the urethra and bladder neck (9) and the suburethral layer of vaginal wall and endopelvic fascia (10) provide a counterforce to the abdominal force that is transmitted to the proximal urethra, thereby closing the bladder outlet. The individual with SUI may have ligaments that are lax and stretched or prolapse of the vaginal walls (11), allowing the bladder neck to descend into a location where increases in intra-abdominal pressure are transmitted only to the bladder and not to the bladder neck and proximal urethra. This produces a significant force for expelling urine.
The magnitude of the pressure exerted on the urethra, which is determined by factors external to the urinary system, such as obesity, chronic lung disease, occupational and recreational stresses, and straining due to constipation, is also crucial in determining the likelihood of SUI (12). These factors not only affect the pressure causing each SUI episode, but may also lead to progressive damage of urethral supports. Numerous other nonmodifiable factors are associated with the onset of SUI, such as genetic factors (13), collagen content (14), race (15), and comorbid disease (16). The remainder of this chapter will discuss management techniques for SUI that affect the modifiable factors related to the onset of SUI. The theoretical rationale and current evidence supporting each intervention will be presented. When applicable, suggestions for improving compliance will also be discussed.
PELVIC FLOOR MUSCLE EXERCISE
PFM exercise, training of the large levator ani and external urethral and anal sphincter muscles, is the most commonly prescribed intervention for the management of SUI (17). The PFM is composed of striated muscle. The levator ani is composed of 70% slow twitch/type I fibers, which use aerobic oxidative metabolism, and 30% fast twitch/type II fibers, which use anaerobic glycolytic metabolism (3,17). Contraction of both muscle types is necessary for normal function. The slow twitch fibers assist in maintaining passive continence, providing pelvic organ support, and are an important part of the postural support system. The fast twitch fibers provide rapid, forceful contractions in response to sudden increases in intra-abdominal pressure, such as with a cough, sneeze, laugh, or lifting maneuver. Interestingly, the slow twitch fibers of the levator ani form are the only weight-bearing muscles in the body whose fibers are oriented transversely.
Individuals with SUI have been shown to have statistically significant differences in PFM function versus continent women (i.e., relative thinning and weakness of the PFM). Continent women were found to have greater PFM thickness than incontinent women as measured by perineal ultrasound (18,19). Mean superficial PFM thickness in healthy women with no history of urinary incontinence or urogynecologic dysfunction was 7.15 mm at rest and 9.41 mm during an active contraction versus 6.34 mm at rest and 8.20 mm during an active contraction in the incontinent women. Greater muscle thickness has been associated with greater strength as measured by vaginal squeeze pressure. Continent women were also found to have a greater squeeze pressure than incontinent women, 39.5 cmH2O versus 32.0 cmH2O, respectively (18,19).
Various factors may affect the strength and thickness of the PFM. When PFM strength was measured in women who had delivered vaginally or via cesarean section and in nulliparous women, PFM strength was significantly lower in the vaginal delivery group compared with the cesarean delivery and nulliparous groups (20). In addition, women who had an episiotomy had significantly weaker pelvic floor muscles at 8 weeks postpartum as compared to women with a spontaneous laceration following vaginal delivery, women who had an elective cesarean, and those with an intact perineum
following vaginal delivery (21). The women who had an elective cesarean showed no significant decline in PFM strength from 36 weeks gestation to 8 weeks postpartum. Women in all three vaginal delivery groups had a significant decline in strength as measured by the vaginal cone weight in grams of the heaviest cone able to be retained for 1 minute with the patient in standing and walking.
following vaginal delivery (21). The women who had an elective cesarean showed no significant decline in PFM strength from 36 weeks gestation to 8 weeks postpartum. Women in all three vaginal delivery groups had a significant decline in strength as measured by the vaginal cone weight in grams of the heaviest cone able to be retained for 1 minute with the patient in standing and walking.
PFM function can be defined qualitatively by the tone at rest and the strength of a voluntary contraction as strong, weak, or absent or by a validated grading system (e.g., Oxford 1-5) (22). A PFM contraction may be assessed by visual inspection, palpation (external and/or internal), electromyography, real-time ultrasound, or perineometry (23). Factors to be assessed include strength, duration, displacement, and repeatability. These variables will determine the personalized exercise prescription. The PFM does not function in isolation and may have function well beyond our current understanding. Studies have demonstrated that the PFM contracts prior to postural movements along with the transversus abdominis and multifidus to provide important trunk stability to facilitate limb movement. The PFM also cocontracts with the obturator internus, another pelvic muscle, which is an external rotator of the hip joint (24). These relationships may also be utilized when designing exercise protocols to maximize PFM recruitment, facilitate a contraction in those with significant PFM weakness, and identify sources of coexisting dysfunction. Table 13.1 gives tips for identifying an ideal PFM contraction.
PFM exercises may aid in the management of SUI through an increased ability to generate urethral resistance by increasing periurethral muscle tension via a learned program of neuromuscular practice (25). PFM exercises are participatory, proactive, simple, noninvasive, free of side effects, and cost-effective and do not limit more complex options for future treatment. These exercises do, however, require time, effort, and continued practice to produce maximum benefit and continued urinary continence (26). The Cochrane Incontinence Group (27) concluded that PFM training was consistently better than no treatment or placebo treatment for SUI and should be offered as a first-line conservative management to women. This recommendation is based on a number of randomized controlled trials that have examined the effectiveness of PFM exercises in the management of SUI in various populations (28, 29, 30, 31).
Participation in a PFM exercise program also results in successful long-term outcomes. With a follow-up of at least 1 year after initiation of PFM exercises, 50% of patients previously referred for incontinence surgery avoided the need for surgical intervention (32). A majority of the women (2/3) who chose a PFM exercise program over surgical intervention remained satisfied with their outcome and were not interested in pursuing surgery 5 years later (33).
TABLE 13.1 Tips for Identifying an Ideal PFM Contraction | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
There has been significant debate, however, as to the most effective way to train the PFM to prevent leakage. Various studies have confirmed that specific exercise instruction is critical to ensure proper performance of a PFM contraction. Performance of a PFM contraction provides little visual or proprioceptive feedback to the person attempting the contraction as there is limited joint motion. Therefore, subjects often report they are unsure if they are performing the PFM contraction correctly. Bump et al showed that more than 50% of stress incontinent women who received verbal instruction alone were performing the PFM contraction incorrectly (34). About half of these women were performing a Valsalva maneuver using contraction of the rectus abdominis muscle, actually promoting versus preventing the loss of urine. These findings were confirmed in a recent study in which perineal ultrasound was used to evaluate the bladder neck during a PFM contraction. During attempts to perform an elevating PFM contraction, 17% of continent and 30% of incontinent
women performed an activity that resulted in bladder neck depression (35). This inherent confusion regarding proper performance of a PFM contraction suggests that intervention from a knowledgeable health care professional is needed to ensure proper performance.
women performed an activity that resulted in bladder neck depression (35). This inherent confusion regarding proper performance of a PFM contraction suggests that intervention from a knowledgeable health care professional is needed to ensure proper performance.
A thorough review of the literature reveals numerous different PFM exercise protocols for the management of SUI. However, based on exercise training of skeletal muscles elsewhere in the body, many physical therapists recommend training sessions three or four times per week for at least 15 to 20 weeks, with three repetitions of eight to ten sustained PFM contractions lasting 6 to 8 seconds each time (10). Incorporation of exercises that indirectly recruit the PFM along with the abdominals and the other key muscles of the spinal stabilization system may also be beneficial (36). For many years, women were instructed to palpate their abdomen during performance of PFM exercises and advised to avoid any contraction of the abdominal musculature. Recent studies have demonstrated that contraction of the deepest layer of the abdominals, the transversus abdominis, should occur with a PFM contraction (37) and actually enhances the PFM contraction when patients are instructed to contract both muscles simultaneously.
Another common method of PFM training that has been popularized in the media is instruction to start and stop the stream of urine during normal voiding. This is no longer recommended because of the potential interruption to normal voiding control mechanisms, leading to incomplete bladder emptying and increased risk of bladder infection. This activity should be used only as a method for patients to test their PFM strength and should be limited (once a week maximum).
Numerous devices are available to aid in the instruction and performance of PFM exercises. Clinicians often find other modalities necessary for education, motivation, and compliance. There is limited evidence available to support the addition of biofeedback, intravaginal resistance devices, or electrical or magnetic stimulation of the pelvic floor over PFM training alone (38). These specific modalities will be discussed later in the chapter.
Noncompliance with a PFM exercise program is quite common. The most common reason cited is forgetting to do the exercises (39). Therefore, some sort of reinforcement is important to improve exercise compliance in women with SUI referred for PFM training. In one study, all women received (a) education regarding causes and treatment of SUI, (b) a PFM diagram, (c) detailed verbal instruction about identification and contraction of the PFM, (d) confirmation using biofeedback, (e) a PFM exercise sheet informing patients to exercise 10 minutes twice a day, and (f) verbal encouragement to perform the exercises for 10 minutes twice a day (26). In addition, the experimental group received an audiotape that reinforced the PFM exercise instruction and provided 10 minutes of PFM exercise coaching. At the 4- to 6-week follow-up, only 65% of the control group reported exercise compliance as compared with 100% of the experimental group. Only 12% of the control group performed the exercises twice per day as instructed compared to 71% of the experimental group. The experimental group performed the exercises three times longer than the control group, on average 15.8 minutes per day as compared to 5.4 minutes. A majority of the experimental group (51%) cited the tape as a reminder to do the exercises.
In another study, subjects had weekly visits with a nurse practitioner to answer questions and encourage PFM exercise training. The overall adherence rate in that study to the prescribed home program was 95.4%. Compliance with a PFM exercise program is challenging yet possible. The practicality of compliance measures in clinical practice needs consideration (40).
Who is the ideal candidate for PFM exercises? Studies have shown that PFM exercises are effective in women with complaints of SUI following vaginal delivery (41). A large prospective cohort study attempted to identify the optimal candidate to benefit from PFM exercises to manage SUI symptoms (42). The results of the study suggested that women with more severe SUI (over two pads per day) and longer duration of symptoms (more than 5 years) were more likely to fail conservative management and require surgery. The results of this study raise the question: Would these patients have been able to prevent surgery if PFM exercises had been initiated earlier (before 5 years)? To date, no other consistent variables have been established to identify patients most likely to benefit from PFM exercises.
PFM exercise may also be a strategy to prevent SUI. A Cochrane review performed in 2002 (27) identified 13 randomized trials of PFM training by women specifically to prevent incontinence. However, many of the studies included women with some urinary incontinence symptoms and therefore cannot be considered purely preventative studies. Three of seven trials in childbearing women report less urinary incontinence after pelvic muscle training compared with control subjects 3 months postpartum, whereas four trials found no difference. In one trial, benefits seen at 3 months were no longer present at 12 months.
Therefore, at this time there is not enough evidence to determine whether PFM training can prevent SUI.
Therefore, at this time there is not enough evidence to determine whether PFM training can prevent SUI.
BIOFEEDBACK
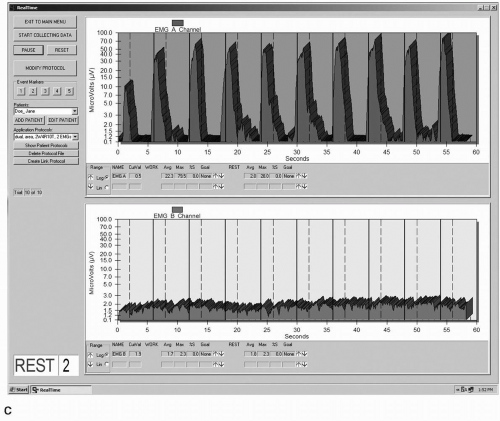
Biofeedback as applied to the stress urinary population has been defined as “a training technique that aims to reverse urinary incontinence by teaching patients to alter the physiologic responses that mediate urine loss” (43). Biofeedback can be achieved through a variety of methods, ranging from a simple offering of verbal cues during palpation of a PFM contraction to more complex techniques involving equipment such as electromyography (EMG), which monitors electrical activity of muscle during contraction and relaxation (Fig. 13.1), or manometry, which measures pressure generation by a muscle contraction (Fig. 13.2). Biofeedback is not an intervention that is intended to be used in isolation, but is best used in conjunction with other behavioral techniques.
Arnold Kegel was the pioneer in the use of biofeedback as an intervention for the management of SUI (44). In 1956 he reported a 90.56% success rate among 455 women who completed a program of PFM exercise utilizing a vaginal perineometer. He described a number of benefits of the use of the perineometer when performing PFM exercise, which include:
The ability to correctly identify the pubococcygeus muscle
The ability to validate that the patient is performing the PFM contraction correctly. This can be reinforced during every exercise session. Kegel believed that a correct contraction with good quality was much more important than a strong contraction.
There are differing opinions and scientific findings reported in the literature as to the benefits of EMG and/or perineometer biofeedback to enhance PFM training. There are a number of reports that describe the benefits of biofeedback training during the initial phase of PFM training (45, 46, 47), but there is controversy as to the long-term benefit of a PFM exercise program that is complemented with biofeedback. The Clinical Practice Guidelines published by the Agency for Health Care Policy and Research (2) assigned a strength of evidence rating A to the evidence supporting biofeedback as an intervention for SUI. The intervention was supported by evidence of properly controlled trials.
The reduction in stress incontinence episodes with biofeedback-assisted pelvic floor exercise programs reported in selected studies ranges from 55% to 80% of study participants (47, 48, 49, 50, 51, 52, 53). Six of these studies compared groups performing PFM exercises with and without the assistance of biofeedback. In most of the studies, there was no significant difference in the amount of urine leakage between groups. Only one study (50) reported significantly less urine leakage in the group that utilized the biofeedback. A systematic review by Berghmans et al (47) supported the results of the majority of the studies. The authors concluded that PFM exercise with biofeedback is no more effective than PFM exercise alone. Conversely, a meta-analysis of the studies in the systematic review reported a trend in favor of biofeedback-assisted exercise programs.
There may be other benefits to biofeed-back-assisted PFM exercise that may influence urine leakage. Aksac et al (53) reported that the biofeed-back-assisted group demonstrated a significantly greater improvement in PFM strength as measured with perineometry (a type of manometry), although there was no significant difference in leakage reduction between the biofeedback and nonbiofeedback training groups. Burns et al (49) reported that the biofeedback group had a significantly greater increase in EMG scores with quick contractions than the nonbiofeedback group. These scores were found to negatively correlate with urine loss.
Therefore, biofeedback may be a valuable tool to use as an adjunct to PFM exercise in certain patients, but there is not significant evidence that it is superior to PFM exercise alone. The success of the intervention may also be “highly dependent on the knowledge and skill of the health care provider” who is directing the treatment (2). This may be the rationale behind the 2001 Medicare ruling regarding reimbursement for biofeedback as an intervention for patients with SUI: biofeedback for the treatment of SUI is a covered treatment in cognitively intact patients only if the patient has failed a 4-week trial of pelvic floor exercises. Many clinicians find the addition of biofeedback to be especially helpful when patients are struggling to perform a pelvic floor contraction or if they are particularly weak or kinesthetically challenged. Improved PFM recruitment has been reported following as few as six sessions of biofeedback training (47). Early success with exercise performance may also serve to motivate the patient to comply with the home exercise program.
EMG or manometric training is usually carried out through the use of vaginal or rectal sensors. The use of vaginal and rectal sensors is contraindicated in the presence of an active infection, pregnancy or less than 6 weeks postpartum, untreated atrophic vaginitis, complaints of pain with insertion of the sensor, recent pelvic or rectal surgical procedure, or if the woman is having her menstrual period (54). Surface electrodes may be an option to replace the internal sensor when contraindications for internal sensor use exist or given personal preference. Optimal monitoring of the PFMs is done with the surface electrodes placed lateral to the anus and medial to the ischial tuberosities.
ELECTRICAL STIMULATION
Electrical stimulation has been proposed as an effective means of activating the PFM in women with SUI (Fig. 13.3). Pelvic floor electrical stimulation can be administered with a single-user vaginal probe or with external surface electrodes, often placed suprapubically and over the sacrum. A high-frequency setting is used (usually between 50 and 100 Hz) to elicit a contraction of the smooth and striated muscles of the pelvic floor (55). Therefore, electrical stimulation may be utilized to provide assistance with active training of the PFM, with the goal of improving the urethral closure mechanism. The use of electrical stimulation may be of greatest value in patients with extreme weakness or those with difficulty eliciting a voluntary contraction of the PFM (29). Although use of electrical stimulation can be limited to clinical use, patients are generally issued a home unit so that training with the stimulator can be completed daily, one or two 20-minute sessions per day.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree