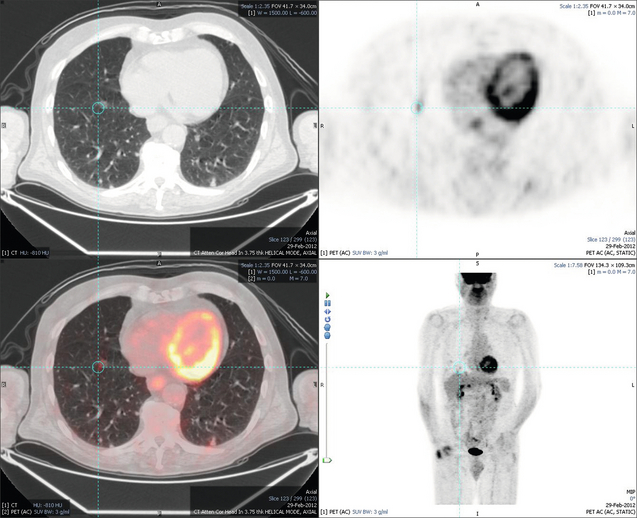
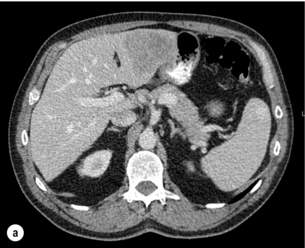
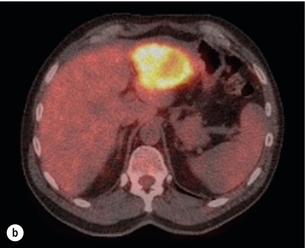
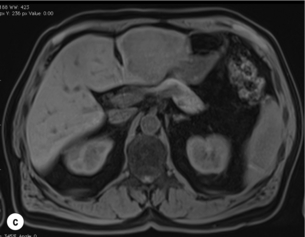
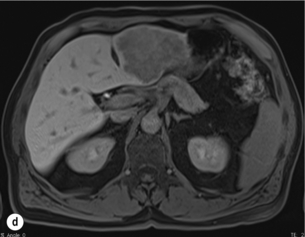
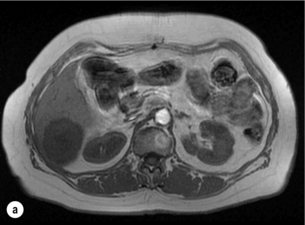
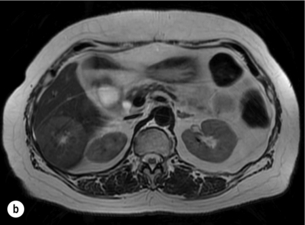
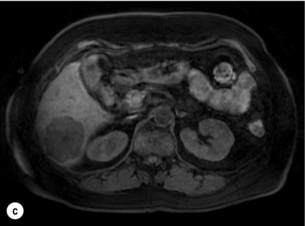
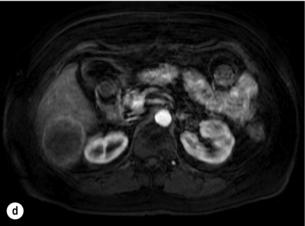
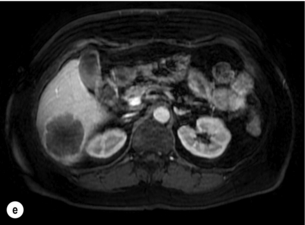
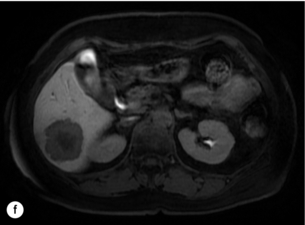
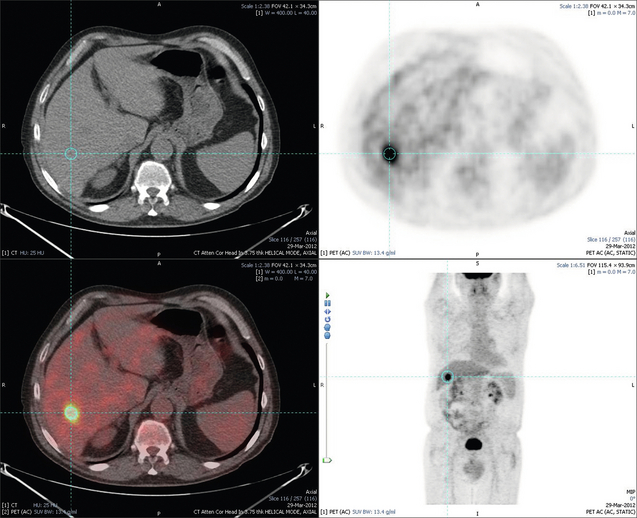
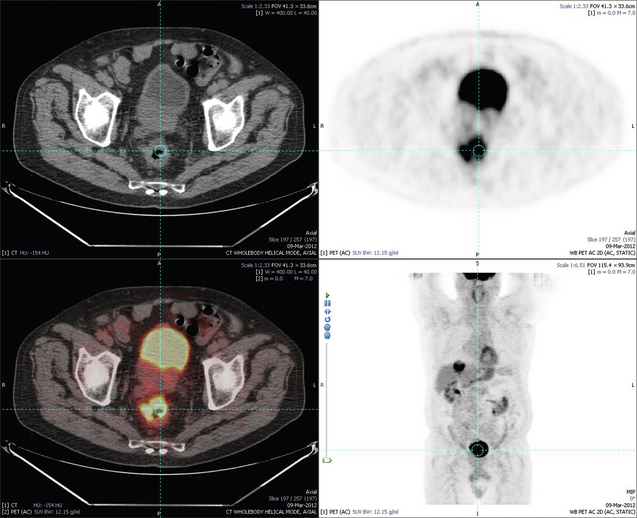
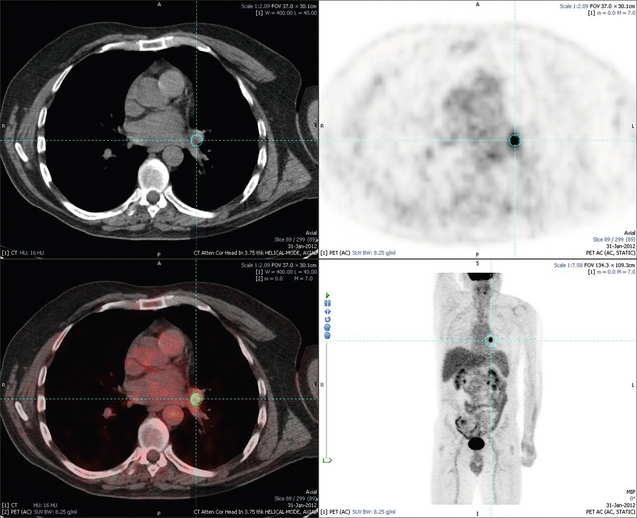
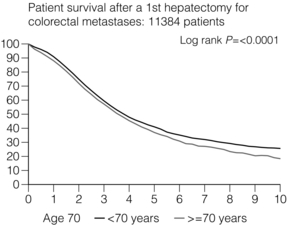
6 Colorectal cancer is the commonest gastrointestinal malignancy and the second commonest cause of cancer death in Western society. Worldwide there are 1.2 million new cases and 608 000 deaths annually.1 The liver is usually the first site of metastatic disease and may be the only site in 30–40% of patients with advanced disease.2 At the time of initial diagnosis of colorectal cancer, 20–25% of patients will have detectable liver metastases. A further 40–50% will develop liver metastases, usually within the first 3 years of follow-up after successful resection of the primary tumour.3 Without treatment, the median survival for colorectal liver metastases (CRLMs) is just 6–8 months, varying with the extent of disease at presentation. The prognosis is best for those whose metastases are isolated to a single lobe of the liver or are limited in number.3 However, even for the best prognostic groups, very few survive 5 years without treatment. Surgery is the only treatment that offers the prospect of cure for CRLMs. Traditionally, only 10–20% of patients were considered suitable for attempted curative resection; the remaining patients were offered palliative and symptomatic treatment. On the detection of colorectal liver metastases it is recommended that patients should be fully staged prior to any planned chemotherapy, and the staging and management plan should be coordinated by a specialist multidisciplinary team.4 Individual imaging techniques used in preoperative staging have different strengths and weaknesses, but consensus is now emerging on the optimal choice of technique and the sequence with which it should be employed.5,6 Imaging techniques are often complementary in the management of colorectal liver metastases, and multiple imaging modalities are often employed. CT is considered a standard of care for all patients identified to have hepatic metastasis.4 Intravenous iodinated contrast media should be used routinely. This helps characterise liver lesions based on their enhancement patterns during the various phases of contrast circulation in the liver.7 During the portal venous phase, normal liver parenchyma usually enhances intensely while liver metastases (with their dominant arterial supply) appear as relatively hypodense hypovascular lesions. In small-sized liver metastases, arterial dominant phase imaging may be useful to detect faint peripheral rim enhancement. Delayed images should be obtained 4–5 minutes after contrast injection. This is helpful in differentiating metastases from benign liver lesions, particularly a haemangioma.8 Whilst CT is considered a standard of care, it has limitations, including the need for a high radiation dose and low sensitivity for the detection and characterisation of lesions smaller than 1 cm (Figs 6.1–6.6). Figure 6.1 (a) CT image in the portal-venous phase demonstrating a hypodense colorectal liver metastasis occupying segments 2 and 3. (b) PET-CT image of the same metastasis demonstrating high uptake of FDG. (c) T1-weighted MRI image of the same metastasis demonstrating a typical hypodense colorectal metastasis. (d) T1-weighted MRI image following primovist contrast administration. Evidence of contrast take-up within the liver and excretion within the common bile duct is observed. No evidence of contrast take-up within the metastasis can be seen. Figure 6.2 (a) T1-weighted MRI image without contrast demonstrating a typical hypodense metastasis in segment 6. (b) T2-weighted MRI image of the same metastasis, where the metastasis is brighter than the surrounding liver. Evidence of central necrosis is seen as a brighter central area of the metastasis. (c) T1-weighted MRI image with fat suppression before contrast administration. (d) T1-weighted MRI image with fat suppression in the arterial phase following primovist contrast administration. The metastasis demonstrates typical rim enhancement. (e) T1-weighted MRI image with fat suppression following primovist contrast administration in the portal venous phase. Good contrast take-up within the liver is observed. (f) T1-weighted MRI image with fat suppression 20 minutes following primovist contrast administration. No evidence of contrast take-up within the metastasis is seen. Evidence of contrast excretion within the gallbladder, common bile duct and kidney can be observed. Figure 6.3 Images extracted from a PET-CT scan demonstrating CT, PET and fused PET-CT images of a liver metastasis in the right liver. Figure 6.4 Images extracted from a PET-CT scan demonstrating CT, PET and fused PET-CT images of a PET-positive primary rectal cancer. Evidence of a left lobe liver metastasis can be seen in the bottom right image. Figure 6.5 Images extracted from a PET-CT scan demonstrating CT, PET and fused PET-CT images of a PET-positive nodal mass in the left superior mediastinum. MRI is a highly effective imaging modality for detecting and characterising liver lesions and provides high lesion-to-liver contrast without using ionising radiation. Typically, CRLMs show low signal intensity on T1-weighted images and moderately high signal intensity on T2-weighted images with fat suppression. Gadolinium, the most commonly used MRI contrast agent, behaves similarly to the iodinated contrast agents used in CT. Liver-specific contrast media such as superparamagnetic iron oxide (SPIO), gadoxetic acid (Primovist®) and Mangafodipir trisodium (Mn DPDP, Teslascan) are not taken up by colorectal hepatic metastases, so may aid in the detection of CRLMs.9,10 These agents are of particular value in the characterisation of liver lesions that are either small or indeterminate on other imaging modalities.8,10 Whilst the benefits of MRI are evident, it does have a number of limitations. MRI has a low sensitivity for detecting extrahepatic disease in the peritoneum and chest, and takes longer to perform than contrast-enhanced CT. There are also a number of contraindications to MRI, including patients with pacemakers, implantable cardiac defibrillators, cochlear implants and metallic orbital foreign bodies.10 However, it can be used safely in patients with allergies to iodinated contrast agents (Figs 6.1 and 6.2). PET has emerged as an important diagnostic tool in the evaluation of CRLMs. Colorectal malignancies are often metabolically active and therefore have a greater glucose uptake relative to that of surrounding normal tissues. This can be identified with [18 F]fluoro-2-D-glucose (FDG-PET). This modality is highly sensitive, especially when combined with CT.11 PET-CT is often used in the preoperative assessment of CRLMs, often with the aim of identifying irresectable extrahepatic disease that would make liver resection futile.12 It can sometimes be difficult to differentiate between malignant tissue and other metabolically active tissue, e.g. inflammatory tissue due to infective or postsurgical causes.12 Mucinous colorectal metastases may also prove difficult to detect due to reduced glucose uptake.13 Other disadvantages of PET include high cost and limited sensitivity for lesions smaller than 1 cm (Figs 6.1 and 6.3–6.6). The yield of laparoscopy for detecting unresectable disease varies from 6% to 36%.13–19 Staging laparoscopy cannot be performed in 6–16% of patients due to adhesions from previous surgery.13–19 One study suggested that staging laparoscopy had greater value in those patients with a higher clinical risk score (CRS).20 The CRS ranges from 0 to 5 based on the presence of the following characteristics: node-positive primary tumour, prehepatectomy carcinoembryonic antigen (CEA) greater than 200 ng/mL, more than one liver tumour, liver tumour size greater than 5 cm and disease-free interval of less than 1 year. In a Memorial Sloan Kettering Cancer Center study,20 only 4% of patients with CRS of 0–1 were irresectable and none were identified as unresectable at preoperative laparoscopy. At scores of 2–3, 21% of lesions were unresectable and only one-half were found at laparoscopy (yield of 11%). The highest yield was at scores of 4–5, where the yield of laparoscopy was 24%. Traditionally, selection of patients for resection has been centred on identifying patients with resectable disease. Recently interest has grown in identifying patients who have a higher operative risk, either from reduced fitness or previously unknown cardiorespiratory comorbidities. Cardiopulmonary exercise testing (CPET) has been shown to be useful in quantifying surgical risk in patients undergoing major hepatobiliary surgery.21 Given that patients over the age of 70 are known to have significantly higher operative risk22,23 and that 50% of patients diagnosed with colorectal cancer are over 70, this technique may have a role to play in the appropriate selection and management of patients undergoing liver resection. If CRLMs are resectable, patients can look forward to a 5-year survival of 40–50% and a 10-year survival of 24%, with age being no barrier to resection if fit (Fig. 6.7). In the past, liver resection was attempted only in patients who had one to three unilobar metastases, preferably presenting at least 12 months after resection of the primary tumour, whose disease was resectable with at least a 1-cm margin of healthy liver tissue and who had no hilar lymphadenopathy or extrahepatic disease. Figure 6.7 LiverMetSurvey. Ten-year survival following hepatectomy for CRLMs comparing patients < 70 years of age with those > 70 years of age. Reproduced with permission. Recent experience has demonstrated that patients outside these narrow parameters can experience long-term survival following liver resection.24,25 Modern criteria for resection are now based on whether a macroscopically complete resection of the disease can be achieved. Instead of resectability being defined by what is removed, resectability is now being determined by what will remain. In 2006, consensus statements from the American Hepato-Pancreato-Biliary Association (AHPBA) and a pan-European group changed the criteria for resection.5,26 The American consensus suggested CRLMs should be considered resectable if (i) the disease can be completely resected (regardless of margin), (ii) two adjacent liver segments can be spared with adequate vascular inflow and outflow and biliary drainage, and (iii) the volume of the liver remaining after resection, i.e. the ‘future liver remnant’ (FLR), will be adequate.5 The European group concluded that criteria rendering patients irresectable included invasion of one branch of the liver pedicle and contact with the contralateral branch, contact with the inferior vena cava, invasion of all three hepatic veins, the presence of coeliac lymph nodes and the presence of non-resectable extrahepatic disease.26 These criteria have already been challenged with long-term survival in patients undergoing nodal resection and resection of metastasis involving the inferior vena cava (IVC).27,28 Resection has also been performed for lesions involving all three hepatic veins, though long-term survival data are not available.29 The 2011 UK national guidance recommended that resection should be offered if a patient is fit enough, and complete resection can be achieved whilst leaving an adequate future liver remnant.4 There are no absolute contraindications to resection issued in this guidance, but in normal circumstances they recommend that contraindications to liver resection are: 1. Non-treatable primary tumour 2. Widespread pulmonary disease 4. Uncontrollable peritoneal disease 5. Extensive nodal disease, such as retroperitoneal or mediastinal lymph nodes Portal vein embolisation (PVE) induces atrophy of the liver to be resected and hypertrophy of the liver that will remain (i.e. increases the future liver remnant), with the aim of avoiding post-resection hepatic insufficiency, liver failure and death. A meta-analysis of 1088 patients confirmed that this technique significantly increased the FLR, making more patients suitable for liver resection.30 The overall morbidity rate was 2.2% without mortality. Following PVE, 930 patients (85%) proceeded to laparotomy. Resection was not performed in 158 patients (17%): in 131 because of inadequate hypertrophy of the FLR and in 27 because of disease progression. Although there is no consensus on what constitutes a safe volume of remnant liver, minimum values of 20–25% for patients with normal livers, 30% following neoadjuvant chemotherapy and 40% in the presence of chronic liver disease have been suggested.5,31,32 PVE also appears to be safe when combined with neoadjuvant chemotherapy.33 Two-stage hepatectomy involves delayed re-hepatectomy after hypertrophy of the residual liver and may be used for large bilateral lesions in which a one-stage resection of all the involved segments would lead to liver failure.34 The first stage involves resection of metastases from the FLR and PVE (or portal vein ligation during surgery), followed by a period of liver regeneration and hypertrophy of the FLR alongside systemic chemotherapy. The second stage is performed 2–3 months later and consists of the major hepatectomy to remove the residual disease. A large series reported 1- and 3-year survival of 70.0% and 54.4%, respectively, in 25 of 33 patients in whom a two-stage hepatectomy could be completed.35 There was no operative mortality; postoperative morbidity was 15.1% and 56.0% after first- and second-stage hepatectomy, respectively. Repeat hepatectomy for patients with colorectal liver metastases is safe and provides survival benefit. A meta-analysis of 21 studies, comprising 3741 patients, showed that there was no difference in perioperative morbidity, mortality or long-term survival between patients undergoing a first or repeat hepatectomy.36 A study looking at 1706 patients undergoing repeat hepatectomy for CRLMs demonstrated similar morbidity and mortality after third and fourth hepatectomies, though 5-year survival decreased from 47.1% for a first resection to 23.8% for a third or fourth resection.37 Resection of tumours involving the hepatic vascular inflow has been described, including portal vein resection and reconstruction, hepatic artery resection and reconstruction (or arterialisation of the portal vein as an alternative).38 Resections of tumours with involvement of the IVC or the three major hepatic veins have also been performed, using techniques such as total hepatic vascular exclusion, in situ hypothermic perfusion and ex vivo (bench) hepatic resection.39–41 These techniques are at the frontier of what is currently feasible and are associated with significant morbidity and mortality. Nonetheless, this aggressive surgical approach may offer hope for patients with hepatic tumours involving the IVC, who would otherwise have a poor prognosis. Extrahepatic colorectal metastases, such as direct diaphragmatic invasion, adrenal metastases and lung metastases, may be resected with curative intent. Reported long-term survival after pneumonectomy for colorectal metastases mirrors very closely that seen after hepatectomy, with most series quoting a 5-year survival of the order of 40–50%, with similar low operative morbidity and mortality.42–45 More recent series have identified 5-year survival approaching 70%46 and showed that repeat resection of pulmonary metastases is also of benefit, with 5-year survival of 42% following second pneumonectomy.47 Other series looking at liver resection in the presence of extrahepatic disease have demonstrated that there is a role for resection of other limited extraheptic disease, including peritoneal, hepatic pedicle nodal disease, aortocaval nodal disease, ovarian and bone metastases.28,48,49 Five-year survival following limited peritoneal and hepatic pedicle nodal disease is quoted at 27% and 26%, respectively.48 Aortocaval nodal disease is associated with worse long-term survival, with a 5-year survival of just 7%.49 Technological innovations in liver surgery have mainly focused on minimising blood loss during transection of the hepatic parenchyma, as blood transfusion is associated with increased postoperative morbidity and mortality, as well as reduced long-term survival.50 Inflow occlusion (Pringle manoeuvre) and low central venous pressure (CVP) anaesthesia minimise blood loss but may cause liver damage by ischaemia and reperfusion injury. Consequently, there has been an interest in devices that facilitate a more bloodless liver transection, obviating the need for inflow occlusion associated with the traditional clamp-crushing technique. The most popular of these techniques include the ultrasonic aspirating dissector (CUSA) using ultrasonic energy, the Hydrojet using a pressurised jet of water and the dissecting sealer (TissueLink) using radiofrequency energy. These techniques were compared in a randomised controlled trial51 and in a subsequent Cochrane review.52 There was little difference demonstrated between the four techniques, though the clamp-crushing technique was found to be associated with faster tissue transection and lower transfusion requirements. The Cochrane review also found an association with fewer infective complications. Both studies highlighted the significantly reduced cost associated with the clamp-crushing technique, and therefore could not advocate the use of newer techniques in standard practice. A further randomised control trial of radiofrequency-assisted versus clamp-crushing transection in 50 patients showed a higher rate of postoperative complications in the radiofrequency group (20%), compared to none in the clamp-crushing group.53 Fibrin sealants have become popular as a means of improving perioperative haemostasis and reducing biliary leakage after liver surgery. However, a randomised study of 300 patients showed no differences in transfusion requirement, overall drainage, incidence of biliary fistula and postoperative morbidity between those receiving fibrin glue application and controls.54 Similar to the newer transection techniques, there is little evidence to justify the fibrin sealants, especially given the financial pressures on healthcare provision. Laparoscopic surgery for hepatic neoplasms aims to provide curative resection while minimising complications. There are no randomised controlled trials assessing the use of laparoscopic hepatectomy and the evidence is based on retrospective series. A meta-analysis of series published between 1998 and 200555 included eight non-randomised studies, reporting on 409 resections of hepatic neoplasms, of which 165 (40.3%) were laparoscopic and 244 (59.7%) were open. Operative blood loss and duration of hospital stay were reduced significantly after laparoscopic surgery. These findings remained consistent when considering studies matched for the presence of malignancy and segment resection. There was no difference in postoperative adverse events and extent of oncological clearance. This paper concluded that laparoscopic liver resection has the potential to reduce operative blood loss and allow earlier recovery with oncological clearance comparable with open surgery. The largest single-centre experience of laparoscopic resection of CRLMs included 83 resections within a series of 133 liver resections.56 Resections comprised 42 wedge excisions, 10 segmentectomies, nine bisegmentectomies, three trisegmentectomies, 30 left lateral segmentectomies, four left hepatectomies, 31 right hepatectomies, three extended right hepatectomies and two caudate lobe resections. The authors reported a median operating time of 210 minutes (30–480 minutes), median blood loss of 300 mL (10–3000 mL) and a median postoperative stay of 4 days (1–15 days). Severe postoperative bleeding occurred in five patients (3.7%), requiring intensive care management or re-operation, and overall serious complications occurred in 16 patients (13%). Microscopically negative margins (R0/R1) were achieved in 96% of patients with CRLMs. In 2008 a group of 45 experts in hepatobiliary surgery participated in a consensus conference and concluded that the laparoscopic approach to liver resection is a safe and effective technique for appropriately trained surgeons.57 The utility of surgical resection of CRLMs is clearly established. Prospective and retrospective studies consistently show 5-year survival rates following liver resection of 30–50%, depending on selection criteria. A major systematic review of surgical resection for CRLMs was undertaken to assess the published evidence for its efficacy and safety and to identify prognostic factors.58 Thirty independent studies met all the eligibility criteria for the review and data on 30-day mortality and morbidity were included from a further nine studies. The best available evidence came from prospective case series, but only two studies reported outcomes for all patients undergoing surgery. The remainder reported outcomes for selected groups of patients: those undergoing hepatic resection or those undergoing curative resection. Death within 30 days of hepatic resection was reported by 24 studies and ranged from 0% to 6.6% (median 2.8%). A further nine studies reported perioperative mortality within an undefined time period (1.3–4.6%, median 3.6%) and two studies reported 60-day mortality (3.4–5.5%). Mortality was not reported in four studies. Cause of death was reported in 15 studies for a total of 103 patients. The commonest specified causes of fatal complications were, in descending order of frequency: hepatic failure, postoperative haemorrhage, generalised sepsis, cardiac failure, multiorgan failure, pulmonary embolism, bile leak and anastomotic leak.58 Staging systems and terminology The present American Joint Committee on Cancer (AJCC) classifies all colorectal metastasis beyond the local lymphatic basin as stage IV colorectal cancer. This does not allow the distinction between patients who are currently incurable, with a prognosis of less than 6 months, from those who are potentially curable. This has led to the call for a new staging system for colorectal cancer that reflects these differing treatment pathways and prognostic outlook.59 The 2003 French guidelines on the management of CRLMs recommended four categories that could be defined: (1) easily resectable liver metastases, (2) resectable liver metastases involving five to six liver segments and/or contralateral major vascular structures, (3) liver metastases that are initially unresectable but may become resectable after chemotherapy, and (4) definitely unresectable.60 Based on the French classification system, the European Colorectal Metastases Treatment group has proposed a staging system:26 • IVa – easily resectable with curative intent at detection (French classification 1); • IVb – technically difficult/borderline resectable at detection (French classification 2); • IVc – potentially resectable after neotherapeutic chemotherapy (French classification 3); • IVd – little or no hope of being rendered resectable with curative intent after conventional chemotherapy (French classification 4); • Va – resectable extrahepatic disease; Other suggested systems include distinguishing between stage IV-R for patients with resectable disease and stage IV-U for patients with unresectable disease.61 Furthermore, stage IV-R could be further divided into IV-Ra (resectable liver only), IV-Rb (resectable extrahepatic only) and IV-Rc (resectable hepatic and extrahepatic). Stage IV-U could be similarly subdivided, after assessment by an experienced site-specific surgical oncologist. A number of scoring systems have been developed that take a different approach to the staging of CRLMs and attempt to classify patients based on clinical prognosis. The most popular of these were produced by Fong et al.,20
Colorectal liver metastases
Introduction
Preoperative staging: the key to selection of candidates for curative treatment
Computed tomography (CT)
Magnetic resonance imaging (MRI)
Positron emission tomography (PET)
Staging laparoscopy
Cardiopulmonary exercise testing
Surgery: the old and the new standards for resection
Surgical strategies to improve resectability
Portal vein embolisation
Two-stage hepatectomy
Repeat hepatectomy
Extreme liver surgery
Extrahepatic colorectal disease
Techniques of surgical resection
Fibrin sealants
Laparoscopic liver surgery: less is more?
Morbidity, mortality and survival after liver resection for CRLMs
Classification of CRLMs
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Colorectal liver metastases