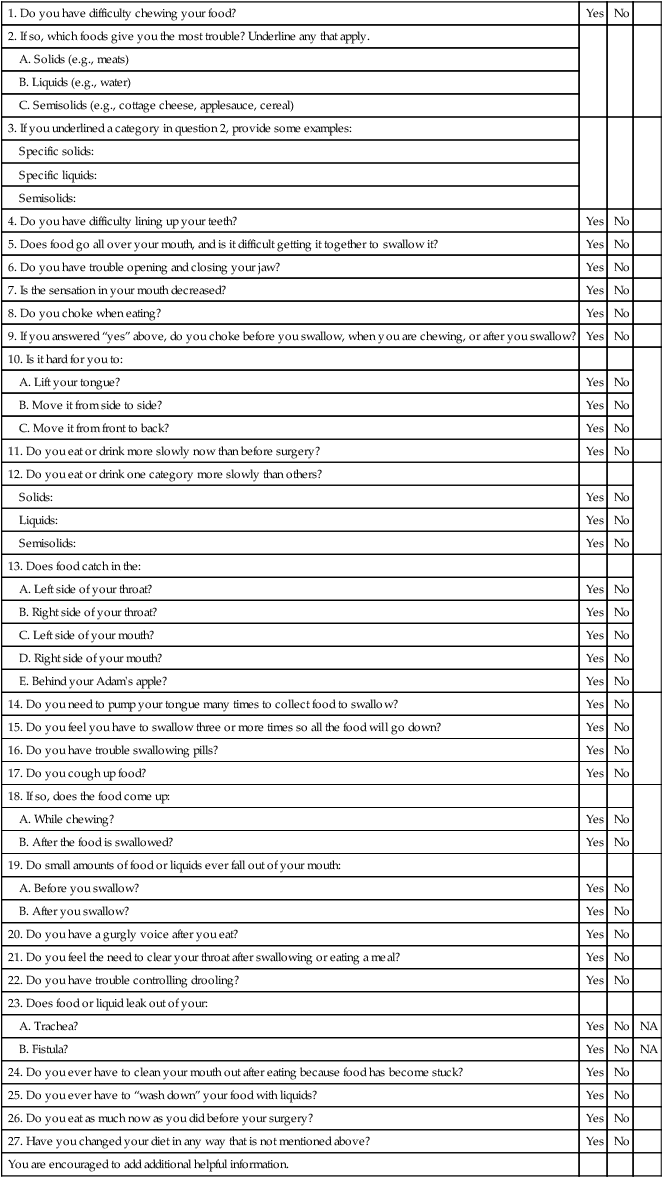
1. Describe the rationale for early detection of swallowing disorders. 2. Review the main components of the clinical evaluation of swallowing in adults. 3. Present the strengths and weaknesses of evaluation protocols. 4. Discuss noninvasive techniques for improving the diagnostic accuracy of the clinical examination. 5. Review standardized tests for dysphagia. 6. Present potential adjunctive measures of swallowing performance. A comprehensive evaluation of a patient with known or suspected dysphagia involves a number of medical and allied health disciplines. Data collected for patients in an outpatient setting who reported dysphagia revealed that the diagnostic process involved an average of 3.5 disciplines per patient.1 Therefore for most patients, the comprehensive evaluation of the patient with dysphagia should be considered a team evaluation. Relevant disciplines were discussed in Chapter 1. For the speech-language pathologist (SLP) the evaluation is intended to assess factors that relate to swallowing function, not to diagnose the underlying disease, although it may either obviate or clarify the need for further studies. In some cases the clinical examination of swallowing confirms a particular diagnosis because the swallowing characteristics are consistent with other aspects of the disease, such as the repetitive tongue pumping in the oral stage of Parkinson’s disease. The clinical evaluation of swallowing often is referred to as the bedside examination. Although the bedside examination encompasses the same procedures, clinical examination is the preferred terminology because the examination is performed in any setting and is not restricted to the bedside. However, modifications in the standard clinical evaluation of swallowing may need to be made at the bedside—often because of poor patient cooperation. The clinical evaluation of swallowing is to be distinguished from the instrumental evaluation (see Chapter 10), which might include tests conducted outside the clinical environment, such as radiographic studies that require special space and equipment. Some patient settings, such as long-term care facilities, lack easy access to instrumental swallowing assessment environments. Patients must be transported to these settings. Therefore clinicians in these settings rely heavily on the clinical evaluation of swallowing to provide diagnostic and treatment information. Settings such as those in tertiary care hospitals can provide the instrumental support for advanced swallowing studies. In this environment the clinician may not always rely totally on the clinical evaluation. Rather, it is viewed as complementary to the instrumental evaluation. Interestingly, there are no prospective data that either support or refute the effect of each practice pattern on patient health outcomes. The clinical evaluation of the patient with dysphagia has three main components: the medical history, the physical inspection of the swallowing musculature, and observations of swallowing competence with test swallows. Logemann2 lists five reasons for performing a clinical (physical) evaluation for a swallowing disorder: (1) to define a potential cause (medical history), (2) to establish a working hypothesis that defines the disorder, (3) to establish a tentative treatment plan, (4) to develop a potential list of questions that may require further study, and (5) to establish the readiness of the patient to cooperate with any further testing. Not all elements of the physical evaluation may be completed because of patient cooperation or performance. In this circumstance, the clinician must rely heavily on the medical history or, if the patient is eating, observations of his or her swallowing ability. Another valuable use of the clinical examination is its use as an outcome measure, either in a research protocol or in clinical practice. Changes in physical status after treatment intervention can be easily measured with a clinical examination with numeric values associated with each finding. Skilled examiners use baseline clinical evaluation data to track dysphagia severity over time in patients with progressive neurologic disease. Practitioners might choose to use an abbreviated portion of the clinical examination of swallowing as a method to screen for or detect dysphagia. Once a high suspicion for dysphagia is established, the entire clinical examination is administered. Early detection of dysphagia is important because complications from dysphagia increase patient morbidity, lengthen hospitalization (health care cost), and may ultimately increase patient risk for death.3 Most “screening” tools for dysphagia do not meet the strict criteria of a screening device. A valid screening tool for dysphagia should be able to detect dysphagia in a large group of patients who are not symptomatic for dysphagia, but who may, in fact, have dysphagia.4 The valid screening tool for dysphagia also should be able to show improved health outcomes as a result of its administration.5 To establish that improvement in health status is a consequence of effective screening, the screening device should be tested in a group with dysphagia who did not receive the screening and a similar group who did.5 Current practice with dysphagia screening tools presupposes that the screening tool is administered because the patient already has a high suspicion for symptoms. A good screening test for dysphagia will have high sensitivity (detecting dysphagia when it is present) and high specificity (determining accurately when it is not present), but it also should be predictive. For patients with dysphagia the screening tool should be predictive of positive health outcomes, such as fewer cases of aspiration pneumonia, better nutritional status, or positive quality of life scores as they relate to eating. Screening devices for dysphagia should be easily administered by any medical specialist in a short time. The clinical evaluation for swallowing as administered by the SLP is designed to provide a much broader perspective of the patient with dysphagia than a screening protocol would provide. The American Stroke Association has called for development of dysphagia screening devices. All patients, regardless of suspicion for dysphagia, will be screened for its presence. Currently, no clinical examination or portions of a clinical examination for swallowing have satisfied the statistical and application criteria needed to be called a screening instrument for dysphagia. One undoubtedly will be developed in the near future because of the importance of early detection. Clinicians recognize that all clinical evaluations of patients with dysphagia are not the same, although clinician preference in selecting items for inclusion are items supported in the literature as discriminative.6 Most clinicians combine data from the medical history, physical examination, and trial swallows.6 The clinical examination for swallow suffers from lack of reliable methods of scoring and inconsistencies in agreement on observations, such as the definition of a wet-hoarse voice.7 McCullough et al.8 found that clinicians can reliably judge only 50% of items commonly administered in a clinical examination for swallow. The most reliable judgments were observation of the presence of tubes, oral motor data, and historical parameters. Inconsistency in recoding data carries the risk of diagnostic inaccuracy, which in turn will affect the treatment plan. Clearly there is a need to standardize the clinical evaluation of swallowing (see section on standardized tests later in this chapter). The physical examination of a patient with dysphagia may begin by asking the patient to describe the symptoms. Some common symptoms are detailed in Table 9-1. Because dysphagia often is secondary to neurologic disease that also may compromise communication skills, not all patients can provide a report of their symptoms. Others may give unreliable or scant information because of cortical deficits. Anecdotal evidence suggests that many patients with dysphagia (particularly esophageal based) do not seek immediate medical attention. Rather, they make changes in their eating habits to accommodate their symptoms, such as chewing food more finely or eliminating troublesome items from their diet. Others know they have difficulty swallowing but cannot describe the specifics of their symptoms. Often it is difficult to remember how long the symptoms have been apparent; this may be from the inherent flexibility of the swallowing tract to accommodate changes in function. Only when these accommodations no longer provide relief or are too difficult to execute does the patient seek medical attention. Some patients may have symptoms of dysphagia but ignore them. For instance, one study of 56 older persons who did not report dysphagia found that a large majority had radiographic abnormalities during swallowing tests. Such abnormalities included poor esophageal motility and pharyngeal weakness.9 TABLE 9-1 Examples of Signs and Symptoms Associated with Dysphagia The literature suggests that asking patients to localize where they believe the problem exists is not always reliable and may not be useful in guiding the tests selected for patient examination, particularly when they report the problem is localized to the neck.10 In one large study of patients who were found to have confirmed esophageal disease, most who pointed to the lower esophagus who had confirmed lower esophageal lesions were accurate. However, a significant number (30%) pointed to the upper neck and chest as the source of their discomfort.11 Other investigators have found that a significant number of patients who described food sticking at the level of the pharynx did have abnormalities at this level; however, the primary source of that abnormality often was found to be in the esophagus.12 This suggests that patients who report dysphagia localized to the neck and pharynx should not only have that specific region investigated, but also should have studies appropriate to the esophagus. Questioning patients about their disorder beyond localization often improves their accuracy. For instance, if the patient localizes the problem to the neck and reports coughing on fluids, the likelihood that the problem is pharyngeal based is high.13 One study found that if patients who complained of food sticking in the region of the neck also reported respiratory symptoms (congestion, wheezing, cough), the sensitivity of dysphagia localized to the pharynx improved.14 Another group of investigators found that subtypes of esophageal disorders (motility vs. obstructive) could be determined by patient report if the patient described a cluster of symptoms, such as heartburn with dysphagia, prior dilatation, pain, and weight loss, than if they reported heartburn alone.15 In general, studies agree that the complaint (dysphagic symptoms) presented by the patient correlates better with the findings when the problem after diagnosis is judged to be severe. The fact that all studies do not agree on whether patient localization is accurate is largely attributable to inadequate numbers of subjects, the potential differences in final classification of the disease type, and the fact that some patients might have undergone other treatments and tests before being enrolled in the study. Some clinicians find it useful to explore a patient’s dysphagic symptoms by questionnaire. A sample questionnaire specific to patients with head/neck cancer that could be completed before their office visit is presented in Box 9-1. This method may help ensure that all relevant questions relating to the patient’s symptoms are addressed by the examiner. It also gives the patient a chance to think carefully about the symptoms before responding. Other patient-specific questionnaires have been developed, including one specifically for stroke (the Burke Dysphagia Screening Test)16 and one for patients with Parkinson’s disease.17 Wallace et al.18 sought to develop a symptom severity assessment tool. Their tool is a 17-point questionnaire designed to evaluate initial dysphagic symptom severity that could be used to judge outcomes after therapy. Questions range from the patient’s difficulty in swallowing various textures to issues of swallow initiation, episodes of choking, and how the disorder interferes with the patient’s quality of life. Regardless of which method is used—patient report to examiner questions or patient responses to a questionnaire—the patient’s subjective complaint may not always fit the objective data gathered in the physical and instrumental evaluation. In another study, subjective complaints of patients with head/neck cancer and oropharyngeal dysphagia were compared with the objective findings from the instrumental examination.19 In general, many of the objective findings did not always support the patient’s complaint. However, in some cases other findings that the patient did not consider important were documented. Jensen et al.19 concluded that subjective complaints may be useful in guiding the examination but should be confirmed with objective data. There have been no comparisons between the standardized dysphagia questionnaire and the structured clinical interview as it relates to diagnostic approach or accuracy. One of the most common complaints from patients with dysphagia is that food or fluids “get stuck.” Most frequently they report that the sticking sensation is in the throat or esophagus. Some patients do not use the word stuck but may use the word fullness. Especially when they localize the feeling of obstruction to the throat, patients often describe their complaint as “a lump in the throat” when eating. The medical term for this feeling is globus. Some physicians have used the term globus hystericus to describe this sensation, because it was once believed that the description of a lump in the throat was usually associated not with organicity but with symptoms of hysteria. Technically, globus hystericus is reserved for patients who complain of a lump in the throat that is relieved by swallowing or talking, not as a cause for dysphagia. The globus sensation is usually relieved by swallowing. However, use of the term globus sensation often is associated with the dysphagic person who reports that food is sticking at the level of the cervical esophagus. Although early investigators reported that they rarely found a cause for the globus sensation (i.e., patients were hysterical), recent reports suggest that with the appropriate battery of diagnostic tests, most who report the globus sensation have identifiable disease.20 Moser et al.21 found that when patients reported the globus sensation with chest pain or heartburn, they were likely to have an esophageal motility disorder. Patients may report a change in their dietary habits that is associated with perceived dysphagia. For instance, patients who describe the globus sensation often have more difficulty swallowing solids than liquids. Classically, those with solid food dysphagia are more likely to have disorders of esophageal origin, whereas those with dysphagia for liquids are more likely to have oropharyngeal dysphagia. This dichotomy, however, may be artificial because it is well known that those with oropharyngeal dysphagia can have dysphagia for liquids and solids, and some forms of esophageal dysphagia evoke complaints regarding liquids and solids.10 When patients report choking on liquids and/or solids, it suggests a more pharyngeal-focused cause, whereas those who report dysphagia for liquids and solids without choking episodes may have a more esophageal-focused cause. Gastroenterologists who suspect the esophagus as the source of dysphagia may use a decision tree such as the one presented in Chapter 7 (see Figure 7-9) to assist in diagnosis. Such a decision tree has not been validated against a large number of patients with confirmed diagnoses; however, the concept is useful because the symptoms related to the diseases represented are well known. Patients are asked questions related to diet (solids vs. liquids), intermittent versus progressive symptoms, and the presence of heartburn. In general, patients with solid food dysphagia are at risk only for more obstructive types of dysphagia in the esophagus. Those who report problems with both liquids and solids more frequently have disorders of esophageal motility. A decision tree for suspected oropharyngeal dysphagia has not been developed, primarily because of overlapping (and therefore nonspecific) symptoms and signs that may be related to many disease entities. Therefore using a decision tree approach based on patient complaints would have little precision in helping establish a diagnosis for those with oropharyngeal dysphagia. Some patients report episodes of gastroesophageal reflux (heartburn) associated with their report of dysphagia. Some patients describe pain or fullness in the chest associated with their reflux. Others may have reflux and dysphagia but may be unaware that they have reflux because the overt symptoms of chest pain, or acid taste, are not present. Not all patients describe episodes of reflux unless questioned by the examiner because they may not relate their reflux symptoms to their dysphagia. This is particularly true when patients describe the globus sensation in the neck because they might not think that reflux in the esophagus could be related to a problem in the throat (see Chapter 7 for a full discussion of gastroesophageal reflux disease [GERD] and dysphagia). Signs are objective measurements or observations of behaviors that people elicit during a physical examination. In a patient with dysphagia who is cooperative, this entails an examination of the cranial nerves relevant to swallowing and, if appropriate, interpretation of any laboratory findings. Examples of patient symptoms and corresponding signs are presented in Table 9-1. Some signs are seen on observation when the patient is eating a meal. Signs and symptoms may overlap. For instance, a patient may report (symptom) liquid going into the nose and food sticking. Both may be seen by the examiner (signs) on the videofluorographic swallowing study. In this circumstance the patient’s symptoms have been confirmed. Figure 9-1 shows a sample medical history form. The form can be used to guide the examiner in gathering important historical elements that may affect the diagnosis and treatment of a person with dysphagia. This information can be obtained from the patient/caregiver or the medical record. Some patients, such as those who have had a stroke and dysphagia, make the connection between their neurologic impairment and their complaint. Others, however, such as those who may have dysphagia after surgery unrelated to the swallowing mechanism, may not make the connection between their surgery and dysphagia. For instance, their dysphagia may be related more to the endotracheal tube placed in the airway during surgery for their knee. A thorough medical history pertinent to dysphagia sometimes reveals important data that either had been ignored by other specialists or may lead to a path of evaluation that had not been considered. The medical history as presented in Figure 9-1 is divided into nine parts: congenital disease, psychiatric disease, surgical procedures, cancer-related procedures, metabolic disorders, respiratory impairment, esophageal disorders, prior evaluations of swallowing, and advance directive status. Neurologic disorders are the most frequent cause of dysphagia. Stroke, head trauma, and progressive neurogenic diseases such as multiple sclerosis, amyotrophic lateral sclerosis, and Parkinson’s disease often precipitate dysphagia. (For a full discussion of neurologic swallowing disorders in adults, see Chapter 5.) It is important to note any medical complications from the disease, particularly any side effects from medications to control the disease that may have adverse effects on swallowing. For instance, a patient who is taking a central nervous system depressant to control seizures may have a concomitant depression in motor function that affects swallowing. Any surgical procedure has the potential to create dysphagic symptoms, particularly if the patient underwent general anesthesia that required the placement of an endotracheal tube through the vocal folds. Damage to the vocal folds could interfere with airway protection, resulting in dysphagia. Any surgical procedure that involves the aerodigestive or respiratory tract should be noted. Patients who have undergone a surgical wrap (fundoplication) of the lower esophageal sphincter (LES) to control GERD may be dysphagic because the wrap is too tight. Patients who have undergone surgical relaxation (myotomy) of the upper or LES should have the circumstances of the outcome explored. Surgery to control cancer in the head, neck, or esophagus is of particular importance. Noting whether a patient’s cancer was treated by chemotherapy or radiation therapy also may help explain common side effects from those therapies that may cause dysphagia (see Chapter 6). Other specific surgical procedures to note include cardiopulmonary surgery, thyroid surgery, surgery in the upper airway, and cervical spine surgery. The risk in these procedures of damaging cranial nerve (CN) X is higher than in other surgical procedures in these regions and therefore places the patient at greater risk for dysphagia (see Chapter 8 for a full discussion).
Clinical Evaluation of Adults
RATIONALE
SYMPTOMS OF DYSPHAGIA
Patient Description
Symptom
Sign
Difficulty chewing
Food spills from lips; excessive mastication time of soft food; poor dentition; tongue, jaw, or lip weakness
Difficulty initiating swallow
Mouth dryness (xerostomia); lip or tongue weakness
Drooling
Lip or tongue weakness; infrequent swallows
Nasal regurgitation
Bolus enters or exits the nasal cavity, as seen on radiographic swallowing study
Swallow delay
Radiographic study identifies transport beyond normal standard
Food sticking
Radiographic study identifies excessive residue in mouth, pharynx, or esophagus after completed swallow
Coughing and choking
Coughs on trial food attempts; material enters the airway on radiographic study
Coughing when not eating
Radiographic study shows aspiration of saliva or lung abnormality
Regurgitation
Undigested food in mouth; radiographic study shows food returning from esophagus to pharynx or mouth mucosal irritation on endoscopy; pH probe study positive for acid reflux
Weight loss
Unexplained weight loss; measurement of weight is below ideal standard
Obstruction
Liquids Versus Solids
Gastroesophageal Reflux
SIGNS OF DYSPHAGIA
MEDICAL HISTORY
Historical Variables
Neurologic Disease
Surgical Procedures
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Clinical Evaluation of Adults