Chapter 16 Chronic Gastrointestinal Bleeding
Introduction
Chronic gastrointestinal (GI) hemorrhage may be overt or occult. Overt bleeding is defined as chronic if it is persistent but not severe enough to cause circulatory compromise. It may be seen in the form of melena or red rectal bleeding. If bleeding is occult, the clinical presentation is anemia, and evidence of occult bleeding is found on testing of the stools. In some patients, chronic hemorrhage may be clinically interspersed with acute episodes.1 Acute GI bleeding is discussed in detail in other chapters.
Chronic GI bleeding includes common clinical scenarios, yet the meaning and diagnostic criteria for the different terms are not well delineated.2 Chronic bleeding from the gut is always significant; in particular, malignant tumors of the gut that are curable may be present. There is no universal agreement regarding the nomenclature of GI lesions that can cause chronic bleeding. Development of new technology, mainly wireless video capsule endoscopy and balloon-assisted enteroscopy has provided an opportunity to revisit the traditional classifications of the source of GI bleeding into upper or lower GI bleeding based on the location of the bleeding either proximal or distal to the ligament of Treitz.3
Some authors propose reclassifying GI bleeding into three categories: upper GI, mid-GI, and lower GI bleeding. Bleeding above the ampulla of Vater, within the reach of esophagogastroduodenoscopy (EGD), is defined as upper GI bleeding; small intestinal bleeding from the ampulla of Vater to the terminal ileum, best investigated by capsule endoscopy and balloon-assisted enteroscopy, is defined as mid-GI bleeding; and colonic bleeding, which can be evaluated by colonoscopy, is defined as lower GI bleeding. A simple classification is presented in Table 16.1. This chapter discusses some of the most frequent causes of chronic GI bleeding. Vascular lesions are an important cause of chronic GI bleeding. They may be solitary, multiple, or diffuse and may exist as isolated abnormalities or be part of a syndrome or a systemic disorder. The internationally accepted term for the endoscopic finding of a mucosally based vascular malformation is angiectasis. The categorization of vascular abnormalities in the GI tract has been inconsistent and a source of confusion.4,5 It can be based on histologic characteristics, gross appearance, or association with systemic diseases. These considerations permit categorization into three broad groups, as follows:
Table 16.1 Causes of Chronic Gastrointestinal Bleeding
| Gastrointestinal Lesions | |
|---|---|
| Within Reach of Upper Endoscope | May Be beyond Reach of Upper Endoscope |
| Esophagitis | Celiac sprue |
| Cameron’s erosions | Crohn’s disease |
| Peptic ulcer disease | Intestinal lymphoma |
| Gastritis and erosions | Small bowel angiodysplasia |
| Duodenitis and erosions | Small bowel tumors |
| Angiodysplasia | Small bowel ulcers and erosions, including NSAID- and other drug-induced lesions |
| Portal hypertensive gastropathy | Small bowel diverticulosis |
| Gastroesophageal cancer | Small bowel varices |
| Gastric or duodenal polyps | Lymphangioma |
| Gastroduodenal lymphoma | Radiation enteritis |
| Partial gastrectomy | Blue rubber bleb nevus syndrome |
| GAVE | Osler-Weber-Rendu syndrome |
| Dieulafoy’s lesion | Small bowel polyposis syndromes |
| Gardner’s syndrome | |
| Amyloidosis | |
| Meckel’s diverticulum | |
| Hemosuccus pancreaticus, hemobilia | |
| Klippel-Trénaunay-Weber syndrome | |
| Colonic Lesions |
GAVE, Gastric antral vascular ectasia; IBD, inflammatory bowel disease; NSAID, nonsteroidal antiinflammatory drug.
Angiodysplasia of the Gastrointestinal Tract
Vascular ectasia of the GI tract, also referred to as angiodysplasia or less accurately as arteriovenous malformation, is a distinct clinical and pathologic entity.6–8 It is the most common vascular abnormality of the GI tract and probably the most frequent cause of lower intestinal bleeding in patients older than 60 years. Although the terms angiodysplasia and arteriovenous malformation have been used synonymously, the term angiodysplasia (Greek angeion, “vessel”; dys, “bad” or “difficult”; plasis, “a molding”), means a poorly formed vessel but with a lesser connotation of congenital origin than with the word malformation. Angiodysplasias are usually distinguished from telangiectasias, which, although anatomically similar, are usually referred to in the context of systemic or hereditary diseases. Because most vascular abnormalities are detected during endoscopy, a classification based on endoscopic appearance has been proposed.9 The classification system recognizes the location, size, and number of angiodysplasias.
Pathogenesis
Angiodysplasias are composed of ectatic, dilated, thin-walled vessels that are lined by endothelium alone or by only small amounts of smooth muscle. Their anatomy has been best shown by studies in which casts of the vessels were made by injecting a silicone material.10 These studies showed that dilated tortuous submucosal veins are the most prominent feature in angiodysplasias. Small arteriovenous communications are present because of incompetence of the precapillary sphincter. Enlarged arteries are also present in bigger angiodysplasias and may be associated with arteriovenous fistulas, which explains why bleeding can be a risk in some patients.
Histologic examination shows dilated vessels in the mucosa and submucosa, sometimes covered by a single layer of surface epithelium. These features are shared by angiodysplasias in the colon and stomach.11 Increased expression of angiogenic factors has been found in human colonic angiodysplasias.12 The pathogenesis of angiodysplasias is not well understood. Four theories have been proposed, as follows:
Epidemiology and Natural History
The prevalence of GI angiodysplasias in the overall population is not well known because asymptomatic individuals usually do not undergo endoscopic evaluation. Angiectases have been seen in 0.2% to 2.9% of “nonbleeding persons”15,16 and in 2.6% to 6.2% of patients evaluated specifically for occult blood in the stool, anemia, or hemorrhage.15–17 Angiodysplasias occur most often in the colon, where they are an important cause of lower GI bleeding, particularly in patients older than 60,18–20 although presentation in patients in their 30s has been described.21 There is no gender predilection.
Clinical Manifestations
Angiodysplasias can remain clinically silent or cause bleeding. The estimated incidence of active GI bleed in patients with angiodysplasia is less than 10%. These lesions may be located throughout the GI tract with a variable rate of bleeding associated with them and presentation ranging from hematemesis or hematochezia to occult anemia.3,22 Bleeding is usually chronic or recurrent and, in most cases, low grade and painless.
Patients with colonic angiodysplasia may present with hematochezia (0% to 60%), melena (0% to 26%), Hemoccult-positive stool (4% to 47%), or iron deficiency anemia (0% to 51%). Melena occurs in at least one-fourth of patients with colonic bleeding. The nature and degree of bleeding frequently vary in the same patient with different episodes, and patients may have bright red blood, maroon-colored stools, and melena on separate occasions. In 20% to 25% of episodes, only tarry stools are passed, and in 10% to 15% of patients, bleeding is evidenced only by iron deficiency anemia, with stools that are intermittently positive for occult blood.23 With effective diagnostic and therapeutic endoscopy, the percentage of patients who have had operations has decreased because most lesions are being identified and treated at the time of the first episode.24 Angiodysplasia can be confidently considered to be a source of GI bleeding in an anemic patient only if it is seen to be actively bleeding. The risk of bleeding in patients who are incidentally found to have nonbleeding colonic angiodysplasia is unknown. The number of lesions and the presence of coexisting coagulopathy or platelet dysfunction may increase the risk for bleeding. Patients who have bled from colonic angiodysplasias are at increased risk for subsequent bleeding.10
Stomach and Duodenum
Angiodysplasias of the stomach have been found to be the cause of blood loss in 4% to 7% of patients with GI bleeding.18,25 Angiodysplasias in the stomach or duodenum are found incidentally in approximately 50% of cases.26
The risk that an incidentally found gastric or duodenal angiodysplasia will subsequently bleed is uncertain. Patients who have bled from gastric or duodenal angiodysplasias do rebleed. This rebleeding was illustrated in a series of 30 patients with gastric or duodenal angiodysplasias; 77% had experienced at least one episode of overt bleeding before diagnosis.18
Small Intestine
Approximately 5% of patients presenting with GI hemorrhage have no source found by upper endoscopy and colonoscopy. In approximately 75% of these patients, responsible lesions can be detected in the small bowel. In patients presenting with obscure overt bleeding (defined as the presence of recurrent melena or hematochezia with normal evaluation by upper endoscopy and colonoscopy), small bowel angiectases are detected in 30% to 60% of examinations.3,27
Colon
The colon is the most common site of angiodysplasias in the GI tract; colonic lesions are most often found in the cecum and ascending colon. In some reported experiences, angiodysplasias of the colon account for approximately 20% to 30% of cases of acute lower GI bleeding, approaching the frequency of acute colonic diverticular bleeding.28 Foutch and colleagues29 noted the prevalence of angiodysplasia to be 0.93% from three prospective studies in which screening colonoscopies were performed in 964 asymptomatic individuals (mean age 61 years).
Conditions with Increased Prevalence
End-Stage Renal Disease
Angiodysplasia is the second most common cause of GI bleeding in patients with end-stage renal disease.30 These lesions account for about 20% of upper GI bleeds and 30% of lower GI bleeds30 and approximately 50% of recurrent upper GI bleeds.31 In a prospective study of upper GI hemorrhage over a 50-month period, vascular ectasia was the etiology of upper GI hemorrhage in 13% of patients with renal insufficiency and was the etiology of bleeding more often in patients with renal insufficiency than in patients with normal renal function.32 The prevalence of vascular ectasia as a cause of upper GI bleeding was related to the duration of renal failure and the requirement of hemodialysis. The lesions can occur anywhere along the GI tract and are usually multiple.31 The reason for the increased prevalence among patients with end-stage renal disease is unknown. One possible explanation is that the lesions are detected more frequently because of the increased risk of bleeding associated with uremia-induced platelet dysfunction.
von Willebrand’s Disease
The association of abnormal von Willebrand’s factor (vWF) is receiving increasing attention in the management of patients with bleeding GI angiectasias. von Willebrand’s disease is a bleeding disorder that results from a qualitative or quantitative defect in vWF. vWF is a complex multimeric glycoprotein present in platelets, plasma, and subendothelium. vWF is essential to platelet adhesion and aggregation at the site of vascular injury. In a study of patients with both bleeding and nonbleeding angiectasias of the GI tract and control patients with colonic diverticular hemorrhage, Veyradier and associates33 showed that most patients with bleeding angiectasias of the GI tract lack the largest multimers of vWF induced by a latent acquired form of von Willebrand’s disease. Because these specific multimers are the most effective in inducing platelet aggregation in high shear stress that is commonly present in the microcirculation of angiectasias, it was concluded their deficiency contributes to active bleeding.
An association between angiodysplasias and congenital or acquired von Willebrand’s disease had been reported previously.34,35 Patients with angiodysplasia found on endoscopy were prospectively tested for von Willebrand’s disease at the Mayo Clinic, but no associations were identified.
Aortic Stenosis
Approximately 50% of patients with bleeding vascular ectasias have evidence of cardiac disease, and 25% have been reported to have aortic stenosis. Bleeding from angiodysplasias in patients with aortic stenosis (Heyde’s syndrome) has been repeatedly reported but is highly controversial.36 In support of this relationship is the observation that bleeding may be improved after aortic valve replacement.37–39 Two possible explanations have been proposed to explain this observation. Patients with aortic stenosis may develop an acquired form of von Willebrand’s disease, which can be reversed after aortic valve replacement.33,40,41 The mechanism is thought to involve mechanical disruption of vWF multimers during turbulent passage through the narrowed valve.42 Patients with aortic stenosis may be more likely to bleed from existing angiodysplasias. The observation that angiodysplasias persist after aortic valve replacement despite the fact that bleeding stops supports this hypothesis.11,43 Another explanation is that existing angiodysplasias may bleed as a result of ischemic necrosis in patients who have a low cardiac output.13,43 However, this explanation is inconsistent with the observations that bleeding angiodysplasias have not been observed with other forms of heart disease associated with a low cardiac output and that a low cardiac output is a late complication of aortic stenosis.
Several retrospective, uncontrolled studies44,45 and a prospective, controlled investigation46 do not substantiate a causative role or association of aortic valve diseases with colonic angiectases. Replacement of the aortic valve for control of bleeding secondary to these vascular lesions is not universally accepted.24 A logical approach to patients with both lesions is to treat the colonic lesion first endoscopically, regardless of whether the patient’s cardiac status warrants surgery. If valve replacement is necessary and endoscopic therapy is unsuccessful, further endoscopic or surgical treatment of the colonic angiodysplasia should be delayed until after cardiac surgery. Further attempts at endoscopic treatment or surgical resection are indicated if bleeding recurs.47 The association between chronic GI bleeding in elderly patients and aortic stenosis becomes more relevant with the advent of transcatheter aortic valve implantation that can be offered even to elderly patients with comorbidities, which could make conventional surgery impossible.48
Progressive Systemic Sclerosis
Vascular lesions are a prominent feature of progressive systemic sclerosis, especially in the CREST variant.49 Angiodysplasias are usually distinguished from telangiectases, which, although anatomically similar, are usually referred to in the context of systemic or hereditary diseases. In patients with progressive systemic sclerosis, sites most frequently involved by telangiectases are the hands, lips, tongue, and face, but gastric, intestinal, and colorectal lesions have been reported. These lesions may be the source of occult or clinically significant bleeding and are best treated by endoscopic coagulation.50
Diagnosis
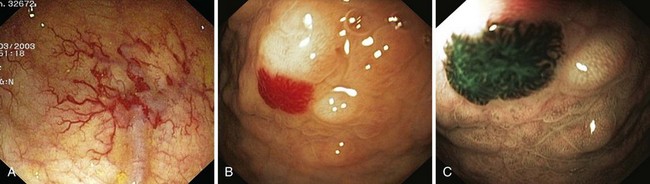
Angiectases have a characteristic appearance of a cherry-red, fernlike pattern of arborizing, ectatic blood vessels radiating from a central vessel (Fig. 16.1). This pattern should be specifically looked for because angiectases may be confused with other erythematous mucosal lesions or with normal vessels (Table 16.2).24,51 Because traumatic and endoscopic suction artifacts may resemble vascular lesions, all lesions must be evaluated immediately on insertion of the colonoscope, rather than during withdrawal. “Anemic halos” are often seen surrounding angiectases of the bowel. Although these “halos” do not differentiate the various types of vascular lesions, they distinguish true vascular lesions from artifacts.47 Newer alternative imaging options, such as narrow band imaging, allow precise discrimination of vascular structures from artifactual mucosal hemorrhage. Punch biopsy samples of vascular lesions obtained during endoscopy are usually nonspecific; the bleeding induced by performing biopsies of these abnormalities is not justified.
Table 16.2 Lesions Confused with Angiectasias on Endoscopy
| Vascular | Nonvascular | Colitis |
|---|---|---|
| Arteriovenous malformations | Trauma | Ischemic |
| Angiomas | Polyps | Infectious |
| Phlebectasia | Adenomatous | Radiation (acute) |
| Varices | Hyperplastic | Inflammatory bowel disease |
| Venous stars | Lymphoid |
Angiectases may be difficult to visualize during colonoscopy in patients who do not have an optimal bowel cleaning. Because the appearance of angiectases is influenced by blood pressure, blood volume, and state of hydration, these lesions may not be evident in patients with severely reduced blood volumes or who are in shock until red blood cell and volume deficits are corrected. Cold water lavage of the colon, as is sometimes done to clean the luminal surface from debris during colonoscopy, reportedly may mask these lesions.52 Meperidine also has been implicated in masking lesions because of a transient decrease in mucosal blood flow. Minimizing use of meperidine and reversal with naloxone to increase the yield of detection have been advocated by some clinicians. Naloxone has been reported to enhance the appearance of normal vasculature in about 10% of patients and to cause angiectases to appear (2.7%) or increase in size (5.4%).53 Reversal of narcotic analgesia may affect the comfort of an examination, particularly if therapeutics are performed.
Some patients presenting with GI bleeding have no source found by upper endoscopy and colonoscopy.3 In these cases of obscure chronic GI bleeding, endoscopic examination of the small bowel has been limited by several factors. The length of the small intestine, in addition to its free intraperitoneal location, vigorous contractility, and overlying loops, confounds the usual diagnostic techniques, including barium studies, endoscopic intubation, and identification of specific sites by special imaging techniques of nuclear medicine scans and angiography. The bleeding rate may be slow or intermittent, not allowing identification by either angiography or radionuclide bleeding scan. Because of the inability to localize a bleeding site in the small bowel, patients with obscure GI bleeding from a small bowel source typically presented with prolonged occult blood loss or recurrent episodes of melena or maroon stool without a specific diagnosis. In this group of patients, previous noninvasive tests, such as small bowel follow-through, radioisotope-labeled red blood cell scan, and push enteroscopy, have had suboptimal diagnostic yields of 20% to 40%. Invasive methods, such as laparotomy or intraoperative enteroscopy, may improve the yield up to 70%.54
For patients with obscure chronic GI bleeding, an early diagnosis of the bleeding site was the exception rather than the norm until more recently with the development of capsule endoscopy and balloon-assisted enteroscopy. The last American Gastroenterological Association Institute medical position statement on obscure GI bleeding considered that for patients with obscure GI bleeding and associated anemia or overt bleeding, repeat endoscopic examinations can be worthwhile.55 Useful adjunctive diagnostic maneuvers include use of a cap-fitted endoscope to examine blind areas, such as the high lesser curve, under the incisura angularis, and the posterior wall of the duodenal bulb; use of a side-viewing endoscope to examine the ampulla in patients with suspected pancreaticobiliary pathology; and use of a push enteroscope to examine the C-loop of duodenum carefully after injection of glucagons. Although the yield is low (6%), repeat colonoscopy may be useful in the setting of prior poor bowel preparation. Use of naloxone may improve the detection of colonic angiectases that were not obvious at the index examination.3
Capsule Endoscopy
The first capsule endoscope, referred to as the M2A (“mouth to anus”), was approved for clinical use late in 2001. The capsule endoscope is a wireless miniature camera that can be swallowed to obtain images of the GI mucosa; the camera contains a light source, batteries, and a radio transmitter. Video images are transmitted via radiotelemetry to a skin surface patterned antenna that allows images to be captured and localizes the relative position of each image in the abdomen.56 In July 2003, the capsule endoscope was approved by the U.S. Food and Drug Administration (FDA) as a first-line tool for the detection of abnormalities of the small bowel, based on evidence provided by a meta-analysis (Fig. 16.2). M2A was renamed PillCam SB (SB means “small bowel”).57
Obscure GI bleeding is the main clinical indication for capsule endoscopy; approximately 70% to 80% of patients undergo capsule endoscopy for this indication. The American Society for Gastrointestinal Endoscopy (ASGE) Technology Assessment Committee concluded that capsule endoscopy provided superior yield compared with radiographic contrast studies and push enteroscopy.58 Two published meta-analyses support the utility of capsule endoscopy in obscure GI bleeding.59,60 The ASGE Technology Assessment Committee also concluded that capsule endoscopy is indicated not only for evaluating obscure GI bleeding, but also for evaluating unexplained iron deficiency anemia.58
The EndoCapsule EC type 1 is another small bowel capsule endoscope, developed by Olympus America (Center Valley, PA). This capsule uses a high-resolution CCD and an external real-time image viewer (External Viewer) monitor.61 A randomized study comparing these two types of capsule endoscope reported a statistically nonsignificant trend for the EndoCapsule to detect more bleeding sources in patients with suspected small bowel bleeding than the PillCam SB.62 Future capsule designs may emerge with expanded capabilities that include fluid sampling, mucosal biopsy, targeted labeling, and controlled movement. With future innovation and study, the indications for capsule endoscopy are likely to expand and become more focused.
Balloon-Assisted Enteroscopy
Push-and-pull enteroscopy with the double-balloon technique (double-balloon enteroscopy [DBE]) for inspection of the entire small bowel was first performed in the Western world in 2003.63 The earliest case report documenting the success of this method was published by Yamamoto and colleagues in 2001.63a
Double-Balloon Technique
The endoscope is introduced into the small bowel within a soft overtube. The balloon at the tip of the endoscope is inflated to hold it in place while the overtube, with deflated balloon, is passed distally until it reaches the tip of the endoscope. Both balloons are inflated, and the entire system is slowly pulled back; this results in an accordionlike sleeving of the small bowel onto the overtube. In the next step, the overtube balloon is kept inflated while the endoscope balloon is deflated and the endoscope is pushed 40 cm deeper into the small bowel. This method is repeated until the enteroscope has been passed as far as technically possible. In approximately 10% of cases, the entire small bowel can be visualized in a single session, typically via an antegrade approach. Carbon dioxide insufflation improves depth of insertion especially during antegrade approaches.64 In most cases, however, antegrade and retrograde examinations are required for the entire small bowel to be visualized. In this setting, the distalmost point in the small bowel that is reached after antegrade passage is tattooed to provide an endpoint during the retrograde examination.
Single-Balloon Enteroscopy
Antegrade DBE can examine three times the length of small bowel as push enteroscopy, with a corresponding increase in diagnostic yield.65 More recently, single-balloon enteroscopy (SBE) was introduced in the United States (Olympus America, Center Valley, PA).66 The single-balloon and double-balloon systems share many features, including scope length, diameter, accessory channel size, and overtube design. The most important design difference between the two systems is that the single-balloon enteroscope does not have a distal balloon on the scope; the only balloon is on the tip of the overtube. As a result, the sequence of steps to advance the scope through the small bowel is simplified in SBE.
Although there are some minor differences between the systems, use of either scope requires a technician to assist with handling of the overtube and balloon inflation and deflation. In addition, both systems require fluoroscopy to monitor scope position and sedation appropriate for prolonged procedures. Early clinical experience using SBE on average reported a depth of insertion (270 cm) and diagnostic yield (54%) similar to DBE with a shorter procedure time.67 Comparison studies between DBE and SBE are inherently difficult to design and carry out. For this reason, accurate comparisons are impossible. Choice of procedure has become a user preference.
Complications
Abdominal pain is common after DBE and can be lessened by the use of carbon dioxide insufflation. Diagnostic DBE has an overall complication rate of 1.7% (perforation 0.3%, bleeding 0.8%, pancreatitis 0.3%).68 The cause of pancreatitis is uncertain. Advancing the overtube and enteroscope into the jejunum before inflating balloons and avoiding excessive tension on the mesentery during push-pull cycles may limit this risk. Therapeutic DBE has a relatively high complication rate of 4.3% (polypectomy bleeding 3.3%, argon plasma coagulation [APC] perforation 1.2%, dilation perforation 2.9%). DBE evaluation of the entire small bowel is possible in 45% to 84% of patients in whom it is attempted, although it can rarely be achieved with antegrade DBE alone. In the evaluation of obscure GI bleeding, DBE has been reported to identify a bleeding source in 53% to 80% of cases.69,70
Three prospective studies compared capsule endoscopy with DBE. Combining the results of the three studies, capsule endoscopy and DBE agreed in 68% of all cases and in 63% of positive cases. A meta-analysis that included these three prospective studies and results published only in abstract form compared diagnostic outcomes between capsule endoscopy and DBE. There was no difference in diagnostic yield between capsule endoscopy and DBE.71 Another meta-analysis reached the same conclusion.72 A new enteroscopy technology consists of a 48-Fr rotating overtube with spiral threads (Discovery SB; Spirus Medical, Inc, Stoughton, MA). The overtube is backloaded on any enteroscope73 or on a pediatric colonoscope.74 After intubation of the stomach with the endoscope, the overtube is rotated clockwise through the upper GI tract until the spiral threads engage in the jejunum; once free in the abdominal cavity, clockwise spinning of the overtube results in rapid pleating of small bowel onto the overtube. In a preliminary report,75 average procedure time for spiral enteroscopy was shorter (32 minutes) than times reported for DBE and SBE. The diagnostic yield is lower (32%) compared with balloon-assisted enteroscopy. The depth of insertion into the small bowel and the overall safety of the large twisting overtube have not been shown.
Summary
Balloon-assisted enteroscopy seems to have superior diagnostic capability compared with push enteroscopy and equivalent yield compared with intraoperative enteroscopy without the associated morbidity of the latter procedure. Although balloon-assisted enteroscopy does not allow visualization of the entire small bowel in one examination, compared with capsule endoscopy, it has been shown to be associated with an equivalent detection rate, has the capability to detect lesions missed by capsule endoscopy, and offers the advantages of therapeutic treatment. In patients with a positive finding on capsule endoscopy, balloon-assisted enteroscopy provides a safe method to achieve a favorable clinical outcome, including significant reduction in recurrent bleeding and transfusion requirements.76 With the advent of balloon-assisted enteroscopy, intraoperative enteroscopy can be relegated to cases in which the success of balloon-assisted enteroscopy is limited by body habitus, the presence of adhesions, or other anatomic factors.3 Most existing trials have focused on the diagnostic value of capsule endoscopy, with few examining the impact on the long-term outcome of obscure GI bleeding. In a 1-year study based on telephone interviews, the negative predictive value of capsule endoscopy for clinical rebleeding was 87%.77 In another more recent report, the negative predictive value was 89%.78 Capsule endoscopy can guide the management of these small bowel lesions.
Similar to other authors,57,80 we79 recommend capsule endoscopy as a first-line investigation over balloon-assisted enteroscopy in view of its convenience, higher chance to visualize the entire small intestine, and similar diagnostic yield. A proposed algorithm for capsule endoscopy in cases of obscure GI bleeding is shown in Fig. 16.3. No test substitutes for good clinical judgment, however, and all small bowel diagnostic studies must be considered in difficult cases of obscure GI bleeding, particularly in a young patient.81
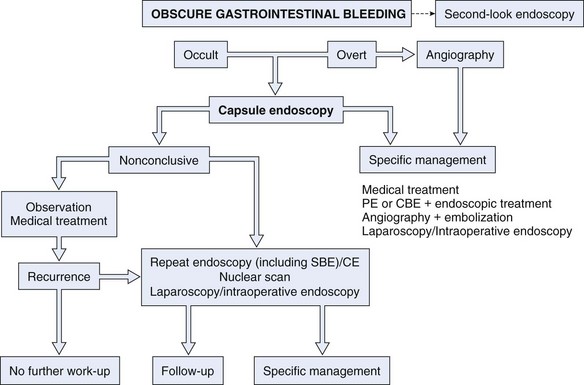
Fig. 16.3 Diagnostic algorithm for obscure gastrointestinal bleeding.
(Adapted from Nakamura T, Terano A: Capsule endoscopy: Past, present, and future. J Gastroenterol 43:93–99, 2008.)
In the setting of obscure overt bleeding, capsule endoscopy should be performed close to the bleeding episode. We recommend that a second endoscopist reread the capsule endoscopy study. We also recommend a second-look EGD with special attention to areas less optimally examined by capsule endoscopy, especially the duodenum, before concluding with a final diagnosis of obscure GI bleeding. Second-look capsule endoscopy has also been suggested for patients with a prior nondiagnostic capsule endoscopy. Bar-Meir and associates82 reported that 7 of 20 patients (35%) who underwent second-look capsule endoscopy had positive or suspicious findings. Another study showed that patients with a nondiagnostic capsule endoscopy test would benefit from a second-look capsule endoscopy if the bleeding presentation changed from occult to overt or if the hemoglobin value decreased 4 g/dL or more.83
Angiography is used to determine the site and nature of lesions during bleeding. It may permit therapy in patients who are bleeding and can identify some vascular lesions even when bleeding has ceased. The three reliable angiographic signs that help diagnose angiectases are a densely opacified, slowly emptying, dilated, tortuous vein; a vascular tuft, and an early-filling vein.84 A fourth sign, extravasation of contrast material, identifies the site of bleeding when bleeding volume is at least 0.5 mL/min but does not contribute to the diagnosis of an angiectasis. Nuclear scan is another diagnostic technique that can be used in selected patients.85,86 Provocative scintigraphy with heparin can enhance diagnosis with further localization of bleeding.87
Helical computed tomography (CT) angiography may provide another method to help diagnose angiectases. Accuracy may be high,88 although studies are needed to understand the role of this technique better in the management of patients with GI bleeding. In a report of 22 patients with obscure GI bleeding, CT enteroclysis was found to be inferior to capsule endoscopy in the detection of potential bleeding lesions such as angiectases in the small bowel.89 In another more recent study performed in 28 patients with obscure GI bleeding, capsule endoscopy detected more lesions (72%) than CT angiography (24%) or standard mesenteric angiography (56%).90 The aggressiveness of the approach should be individualized depending on the clinical circumstances. Evaluation of the small bowel may be deferred in patients with negative upper and lower endoscopy until or unless the patient is bleeding severely enough to require a transfusion.55
Management
Management of bleeding angiectases consists of three phases24: (1) diagnosis; (2) conversion of acute bleeding manifesting as an emergency to elective care by control of the acute hemorrhage; and (3) definitive treatment of the lesions by endoscopic ablation, angiography, or surgical removal. The natural history of colonic angiectases is benign in healthy, asymptomatic individuals, and the risk of bleeding is small.29 It is estimated that only about 50% of colonic lesions ever bleed. There is a risk of bleeding and perforation following attempts at endoscopic obliteration. For all these reasons, in incidentally found angiectases at all levels of the gut, endoscopic therapy is not warranted.91
Pharmacologic Treatment
Iron Formulations
Nonbleeding angiodysplasia detected during evaluation of occult bleeding or iron deficiency anemia should be considered to be causative. In patients with occult bleeding, bleeding from angiodysplasia may be more likely in patients who have multiple lesions and a bleeding diathesis (e.g., anticoagulation). As a result, a graduated approach with primary or adjunctive iron replacement therapy may be initiated, with pursuit of more aggressive therapeutic options guided by the clinical circumstances.91–93 The aims of iron replacement therapy should be to restore hemoglobin levels and mean corpuscular volume to normal and to replenish body stores.
Hormonal Therapy
Estrogen-progesterone combination hormonal therapy has been used to treat patients with angiectases of the GI tract.94,95 The effect, which is not immediate, seems to be estrogen dose–dependent. Hormonal therapy acts by enhancing microvascular circulation, coagulation, and vascular endothelial integrity. The most common combination schedule has been ethinyl estradiol 0.01 to 0.05 mg and norethisterone 1 to 3 mg. This therapy should be used in 6-month courses with pauses to reduce the incidence of adverse effects, mostly secondary to the estrogen component. The results of several prospective, controlled trials examining hormonal therapy have been divergent.96–99 In a long-term observational study, combination hormonal therapy was shown to stop bleeding in patients with occult GI bleeding of obscure origin, likely to have resulted from small bowel angiodysplasias.98 Although uncontrolled studies suggest that combination estrogen-progesterone therapy prevents bleeding episodes secondary to angiodysplasia, the evidence from the largest placebo-controlled trial to date suggests that this therapy is ineffective.100 These authors considered that efficacy of hormonal therapy in these patients remains to be proven by a large, randomized, placebo-controlled trial with long-term follow-up.
Octreotide
Reports of efficacy of octreotide in the treatment of angiodysplasia have been limited to case reports and small series in which a response has been reported in some patients.95,101–103 Octreotide produces vasoconstriction secondary to inhibitory effects on growth hormone and multiple GI vasodilator hormones and markedly reduces splanchnic blood flow. Its antiangiogenic properties have been shown in different tissues (eye, placenta, liver, and GI neuroendocrine tumors); however, applicability and utility in obscure GI bleeding remain unknown.
The dosage of octreotide can be tapered to the lowest quantity that prevents rebleeding. Response is immediate, and the drug can be administered intravenously (50 µg/hr) or subcutaneously (50 to 100 µg two or three times a day).95 Its subcutaneous administration and its longer half-life (90 to 100 minutes) make octreotide superior to somatostatin and allow use in the outpatient setting. A 6-month course of therapy has been used to treat most patients. The first comparative cohort study showing the benefit of long-term octreotide compared with placebo in preventing bleeding from angiodysplasia was reported more recently.104 Compared with placebo, octreotide markedly reduced the risk of rebleeding with a significant decrease in iron requirements, but there were no differences in hemoglobin levels or transfusion requirements between the two groups.
Sandostatin LAR Depot is a depot formulation of octreotide for long-term maintenance therapy currently approved for acromegaly and GI and pancreatic neuroendocrine tumors. Compared with conventional octreotide, Sandostatin LAR Depot is administered intramuscularly once a month with a similar efficacy and safety profile and does not require hospital admission, which makes it an attractive outpatient option for long-term therapy in patients with chronic GI bleeding. The effectiveness of Sandostatin LAR Depot at a dose of 20 mg intramuscularly once a month for obscure GI bleeding has been described in several small series.95,102,105–107 The main disadvantage of this drug formulation may be its cost compared with conventional octreotide. However, in very specific cases, it may prove to be cost-effective. The appropriate dose and schedule required for long-term therapy are unknown.
Thalidomide
Thalidomide is a drug with powerful immunomodulatory, antiinflammatory, and antiangiogenic effects that was withdrawn from the market in the 1960s because of its teratogenicity; it has been reintroduced more recently for the treatment of leprosy, multiple myeloma, and various tumors. Vascular endothelial growth factor (VEGF) has been identified as the key mediator for endothelial vessel formation in early phases of angiogenesis. High concentrations of VEGF result in aberrant angiogenesis with formation of angiectasias that lack a smooth muscle cell layer and are more susceptible to rupture and bleeding. VEGF-dependent angiogenesis is inhibited by thalidomide. Thalidomide is an innovative and promising therapeutic option for GI bleeding associated with angiodysplasia and can be used in refractory cases or when other drugs or therapies are contraindicated.95
Thalidomide is administered orally at a variable dose of 100 to 300 mg/day.95 No significant adverse effects have been reported except transient fatigue; however, thalidomide is contraindicated in patients with peripheral neuropathy and pregnant women and women with childbearing potential because of its teratogenic effects, and it should be used cautiously in patients with cardiovascular or neurologic disorders and hepatic or renal impairment. Owing to its immunosuppressant activity by blocking tumor necrosis factor, use of thalidomide may be also discouraged in patients at risk for infection or chronic infectious disease, especially patients with human immunodeficiency virus (HIV) infection. In all these clinical settings, Sandostatin LAR Depot may be safer than thalidomide.
There are few reports in the literature about the effectiveness of thalidomide for chronic GI bleeding. It was successfully used in a patient with von Willebrand’s disease and life-threatening bleeding caused by small bowel angiodysplasia refractory to other pharmacologic treatments and endoscopic cauterization with argon beam laser.108 It has also been proven effective in controlling bleeding from diffuse idiopathic angiodysplastic lesions in the small bowel.109,110 Bauditz and coworkers111 studied the effect of thalidomide at a dose of 100 mg daily for 3 months in three patients with chronic bleeding from small bowel angiodysplasia as evidenced by wireless capsule endoscopy. Bleeding was controlled in all cases for a median follow-up of 34 months despite drug discontinuation. Repeat capsule endoscopy after therapy revealed a substantial reduction in lesion numbers, size, and color intensity.
Miscellaneous Agents
Antifibrinolytics
Tranexamic acid is a synthetic lysine analogue that inhibits the conversion of plasmin to fibrinogen. It has been used successfully for chronic bleeding from angiodysplasia in patients with end-stage renal failure at doses of 10 to 20 mg/kg every 48 hours; it remains unclear whether long-term therapy or as-needed therapy is preferable during hemorrhagic crises.95,112 The main risk derived from the use of antifibrinolytics is thrombosis, so thrombophilia should be ruled out before prescribing them. Adverse events associated with tranexamic acid may be frequent, and use of these drugs is not supported by randomized controlled trials, which makes antifibrinolytics a last option for chronic GI bleeding. Because of their mechanism of action, antifibrinolytics may have a more important role in the treatment of patients with hematologic disorders.
Danazol
Danazol is an antigonadotropin drug with weak androgenic activity that blocks pituitary secretion of follicle-stimulating hormone and luteinizing hormone, leading to ectopic and normal endometrial tissue atrophy. It has been used for endometriosis and uterine bleeding disorders. Anecdotal reports suggest a partial improvement in patients with GI bleeding and hereditary hemorrhagic telangiectasia.44,45 Cosmetic stigmata (acne, hair loss, mild hirsutism) and uncommon but severe adverse effects (intracranial hypertension, peliosis hepatis, thrombosis, seizures) consign danazol to a secondary role in chronic GI bleeding, to be used when other therapies have failed.
Desmopressin
Desmopressin is a synthetic analogue of the antidiuretic hormone vasopressin that lacks vasopressor activity. It increases vWF and factor VIII levels and enhances hemostasis in patients with defective platelet function. It is indicated as a hemostatic agent for patients with hemophilia A and von Willebrand’s disease, and it can be administered intravenously, subcutaneously, or by intranasal spray. An isolated report showed a benefit of intravenous desmopressin for life-threatening GI bleeding in a patient with hereditary hemorrhagic telangiectasia and vWF deficiency, allowing elective colectomy and bleeding resolution.113
Recombinant Activated Factor VII
Recombinant activated human factor VII has been used mainly in upper GI hemorrhage related to cirrhosis or acute liver failure,114,115 although it has been used in other settings, including refractory bleeding after endoscopic sphincterotomy in patients with preexisting coagulopathy.116 Sporadic reports have shown the effectiveness of factor VIIa in patients with von Willebrand’s disease for chronic GI bleeding secondary to small bowel angiodysplasia or of unknown origin.117 Secondary myocardial and cerebrovascular infarctions have been described while using factor VIIa owing to its marked prothrombotic activity,118 so particular care should be taken in patients with a high-risk cardiovascular profile, and the indication should be assessed for each individual case.
Endoscopic Treatment
Bipolar Or Heater Probe Coagulation
Bipolar or heater probe coagulation is said to be effective for treatment of angiodysplasia,119,120 and these modalities have replaced monopolar coagulation.14,121
Sclerosant
Injection of a sclerosant, such as ethanolamine, has been used to obliterate lesions.122 Epinephrine injection works by volume tamponade of the bleeding vessel and transient vasoconstriction.123 Sclerosants should probably be avoided, however, because of the risk for bleeding from injection site ulceration and perforation.47
Band Ligation
Band ligation has been used to treat angiectasia of the stomach.124,125 The relatively small surface area incorporated into the ligation site (e.g., in GAVE or “watermelon stomach”) and, more so, the expense of the therapy outweigh any perceived benefits. The walls of the small and large intestine above the rectum (especially the right colon) are thinner than the stomach. For this reason, band ligation therapy cannot be advocated owing to the recognized risk for perforation.126,127
Lasers
Argon and neodymium:yttrium-aluminum-garnet (Nd:YAG) lasers have been used in the past.128–130 These techniques require expensive equipment and specific training. More convenient and safer coagulation options have replaced laser therapy except for treatment of “watermelon stomach” (GAVE). APC has become the dominant therapy for GAVE. A few patients are refractory to this therapy. These patients most often respond to treatment with Nd:YAG laser.
Argon Plasma Coagulation
APC is a monopolar electrosurgical procedure in which electrical energy is transferred to the target tissue using ionized and conductive argon gas (argon plasma).131 The plasma beam follows the path of least electrical resistance. This phenomenon permits the argon plasma to be applied both en face and tangentially, allowing treatment of regions that are normally difficult to access. A second-generation APC (VIO APC; Erbe Elektromedizin, Tuebingen, Germany; and Beamer, Con Med, Utica, NY) offering a broader bandwith of APC modes compared with the earlier generators has been introduced more recently to GI endoscopy. The optional coagulation modes (e.g., “forced,” “pulsed,” and “precise”) of second-generation devices provide different coagulation tissue effects. These effects include a continuous energy output, pulsed energy (with high energy per pulse with longer pauses per pulse or a greater number of pulses with a lower energy per pulse with a constant voltage maintained for each pulse option).
APC has been used for various bleeding lesions, including angiectases.132,133 Its great advantage over laser or photodynamic therapy is the limited depth of tissue injury and lower cost. Shallow tissue injury is due to the fact that the argon stream always seeks electrically conductive areas of tissue, avoiding the coagulated zones, which have lost their electrical conductivity as a result of desiccation. Perforation of the cecum has been reported.132 APC seems to be most effective131 in the treatment of vascular lesions (GAVE, angiodysplasias, and Dieulafoy’s lesions).
The utility of obtaining a submucosal saline solution cushion before APC therapy to prevent deep tissue injury has been shown in a porcine model.134 Before APC, it is recommended to collapse the lumen partially while maintaining the treatment site in view. It is important to avoid thinning of the colonic wall with excessive air insufflation because this increases the risk for perforation during therapy, especially in the cecum.123
Cryotherapy
Safety and efficacy of cryotherapy have been reported for treatment of diffuse mucosal lesions of the GI tract.135 The effectiveness of endoscopic treatment of angiectases is difficult to assess owing to the absence of prospective controlled trials. Recurrent bleeding from cecal angiodysplasia seems to be reduced after laser photocoagulation or thermal ablation via heater probe or bipolar electrocoagulation, with long-term control of bleeding requiring more than one treatment.136 Aside from perforation, the main risks of thermal therapy for colonic angiectases are bleeding in 5% of patients and postcoagulation syndrome in 1.7% of cases.120 Endoscopic treatment of vascular lesions in patients known to have coagulation disorders carries an increased risk of procedure-induced hemorrhage.
When treating large lesions, some experienced endoscopists recommend47 to ablate first the periphery of the lesion to create a collar of edema that theoretically reduces the vascular supply to the lesion and diminishes the potential for immediate or delayed hemorrhage. Some clinicians have placed mechanical clips around the margins of large lesions also to reduce the blood supply and facilitate effective coagulation. Others typically target the central portion of the angiodysplasia because ensuing coagulation and edema obstruct the peripheral branches and limit the extent of coagulation needed. Also, for a very large angiodysplasia, epinephrine injection may contract the lesion and reduce the amount and area of coagulation needed for eradication.123 Recurrent bleeding can be expected in approximately 20% of patients with colonic angiectases and in a greater percentage of patients with associated coagulopathies, renal failure, portal hypertension, or additional upper GI vascular lesions.47 It is reasonable to attempt endoscopic therapy in patients with accessible lesions despite various coexisting morbidities that may allow only short-term success. Patients with multiple lesions or an underlying bleeding diathesis are less likely to benefit long-term from endoscopic therapy and are at increased risk of complications, especially delayed bleeding from treatment site thermally induced ulceration. Such patients benefit from any attempts to improve their underlying bleeding tendency.
In preparation for endoscopic ablation of vascular lesions, aspirin, nonsteroidal antiinflammatory drugs, anticoagulants, and antiplatelet agents should be withdrawn at least 1 week to 10 days before the procedure. Care should be taken not to distend the cecum fully because the wall would be thinned further, and the risk of perforation would be increased.24,123 After therapy, patients must be cautioned not to resume full doses of anticoagulants or antiplatelet agents for at least 1 week. Coagulated tissues are at their maximum of thermal injury by 5 to 10 days, and the onset of hemorrhage may be delayed.
Angiography
Localization of active bleeding permits embolization or infusion of vasopressin. Because angiography occasionally causes serious complications, such as arterial thrombosis, contrast reactions, and acute renal failure, its use before definitive surgical therapy has been questioned.137 Angiography should be reserved for patients with life-threatening bleeding, patients who are not surgical candidates, and patients in whom localization of lesions is desired before surgical resection. Intravenous or intraarterial vasopressin infusions through the angiographic catheter successfully arrest hemorrhage from vascular ectasias in more than 80% of patients in whom extravasation is demonstrated.16 The intravenous route seems to be as effective as the intraarterial route when the bleeding is in the left colon, but intraarterial administration is more successful when the bleeding is from the right colon or small bowel. Infarction of the sigmoid colon and severe arterial spasm and lower extremity ischemia have resulted from vasopressin infusions into the inferior mesenteric artery given at the same rate as used in the superior mesenteric artery. These complications may be avoided by infusing less than 0.4 U/min (the dose of superior mesenteric artery infusions), recognizing the lesser blood flow of the inferior mesenteric artery.
Surgery
In the latter two situations, right hemicolectomy is done as an elective procedure after active bleeding is controlled. The entire right half of the colon needs to be removed to ensure that no angiectases are left behind. Recurrent bleeding can be expected in 20% of patients. Subtotal colectomy should be performed only as a last resort in circumstances in which colonic bleeding is strongly suspected but the site and cause are unknown.24 Richter and colleagues16 studied the course of 101 patients with angiodysplasias to determine the natural history and compare the efficacy of medical therapy, endoscopic electrocoagulation, and surgery (right hemicolectomy). Similar rates of recurrent bleeding were observed for medically and endoscopically treated groups during a mean follow-up of 22 months. Surgically treated patients had a frequency of recurrent bleeding less than half that of the other groups.
Hereditary Hemorrhagic Telangiectasia
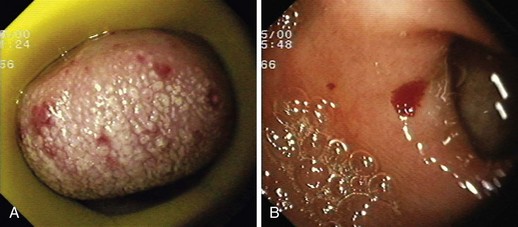
Hereditary hemorrhagic telangiectasia, or Rendu-Osler-Weber disease, is an uncommon, autosomal dominant disorder characterized by telangiectases and arteriovenous malformations that affect many organs, including the skin, periungual areas, lips, oral and nasopharyngeal membranes, tongue (Fig. 16.4A), lungs, GI tract (Fig. 16.4B), liver, and brain,138,139 and can result in bleeding. Lesions consist of irregular, ectatic, tortuous blood spaces lined by a single layer of endothelial cells and supported by a fine layer of fibrous connective tissue. In these vessels, no elastic lamina or muscular tissue is present, so they cannot contract; this property may explain why these lesions tend to bleed.24