13
Cardiovascular Heart Disease in Older Men and the Role of Androgens
Case History
Mr. C.M. is a 50-year-old man who consulted with a view toward testosterone replacement therapy. He is very proactive about his health, and has visited many health centers throughout the United States. He has an extensive cardiac history including hypertension and hypercholesterolemia. He had a heart attack 5 years ago, and underwent angioplasty and a stent placement. He feels well, and has no chest pains at the moment. He states that he does get chest pains off and on, however. He confesses to be short of breath on extreme exertion like running a mile, but has no orthopnea. He has a positive family history of hypertension and coronary heart disease. His current medications include atorvastatin, atenolol, and felodipine. He also is a chronic drinker and consumes two or three beers each day, but does not smoke. On physical examination, the patient was noted to be centrally obese, and his weight was 250 pounds. His blood pressure was 135/100 mm Hg while on medication. He had some signs of hypogonadism, including testicular atrophy but no problems with his sense of smell. Muscle strength was normal and there were no focal neurological deficits. After confirming biochemical hypogonadism, he was put on testosterone gel. He was also prescribed a course of aerobic and strength training exercise. The patient was followed up regularly to ascertain his progress.
Aging and the Cardiovascular System
Due to adequate compensatory mechanisms, the changes affecting the cardiovascular system due to normal aging do not necessarily result in dramatic changes in the heart rate, left ventricular systolic function, or cardiac output at rest. When cardiovascular disease develops, however, its impact is superimposed on the already stressed cardiovascular system, resulting in increased functional impairment, morbidity, and mortality in the elderly population. Both atherosclerotic cardiovascular disease and hypertensive cardiovascular disease have a very high prevalence in the United States and other Western societies. The increase in prevalence is dramatic with age: statistics show that the majority of all cardiovascular deaths in the United States occur in patients older than 65 years of age. More than half of the patients hospitalized annually for acute myocardial infarctions (MIs) are more than 65 years old. Autopsy data from the 1950s and 1960s showed significant coronary artery stenoses in approximately three fourths of men over age 50 years. Congestive heart failure is the most common medical cause for hospital admissions in the elderly. Although recent trends in diet, smoking, physical activity, management of hypertension, and hyperlipidemia may have a significant impact on the prevalence of cardiovascular disease, the magnitude of the change is not known at this time.
Coronary Artery Disease in Men
Although the incidence may have reached a plateau in our Western population, coronary artery disease (CAD) in men continues to be the largest killer by far. By the age of 40, a man has greater than a 3% chance of a coronary event over the next 5 years. Even younger men with multiple risk factors may have this high of a risk. By the age of 50, a man has a 10% risk of a coronary event over the next 10 years. After the age of 40, if stroke is considered along with coronary events, more men die from cardiovascular events than all cancers combined.
Atherosclerotic CAD begins very early in life. At autopsy, 20% of 30- to 34-year-olds have been noted to have 40% stenosis in the left anterior descending coronary artery.1 In fact, fatty streaks have been noted in the aortas of newborns whose mother had severe hyperlipidemia.2 And this is despite the fact that among children and adolescents, the mean total cholesterol is only 165 mg/dL. Only ∼10% of adolescents, ages 12 to 19 years, have total cholesterol > 200 mg/dL.
At this age, obesity, hypertension, and inadequate levels of high-density lipoprotein (HDL) cholesterol are more commonly associated with atherosclerotic streaking. There is not a gender difference, and this is interesting because girls have significantly higher levels of both total cholesterol levels and low-density lipoprotein (LDL) cholesterol from ages 4 to 19 years. Perhaps part of the explanation is that before puberty, there is little gender difference in HDL cholesterol levels.
Following puberty, the synthesis of testosterone in males suppresses hepatic production of apolipoprotein A (ApoA), the precursor of the HDL particle. This leads to a 20% reduction in HDL cholesterol in men compared with women. This difference persists through adulthood and is now thought to explain the gender difference in early heart disease.
Interestingly, beginning at age 50, women catch up with men from the perspective of total cholesterol levels. In fact, at this age a higher percentage of women than men have a total blood cholesterol of 200mg/dL or higher. Granted total cholesterol is not the only risk factor, the American Heart Association (AHA) reports that a 10% decrease in cholesterol levels may result in a 30% decrease in the incidence of CAD. A reduction of total cholesterol in both sexes is therefore universally beneficial in our Western population.
Another impact upon HDL cholesterol and a risk factor for CAD that is exploding in prevalence is the cardiovascular metabolic syndrome. This syndrome affects ∼25% of men by the age of 40 and ∼45% of men by the age of 65. The diagnosis is made if a patient has three of the five factors listed in Table 13–1.
Being overweight is the most common attribute of those patients with metabolic syndrome. With over half of individuals in Western countries being overweight, the incidence of the metabolic syndrome is sure to increase. What is becoming apparent is that adipose tissue is an endocrine organ, especially adipose tissue located at the waist in men, the so-called central adiposity. Although adipose tissue is supposed to produce leptin when there is sufficient energy stored, which is intended to decrease the appetite, this mechanism does not function properly in many individuals. Another functional problem with central adipose tissue it that it is exquisitely sensitive to catecholamines. With the slightest exposure to catecholamines, it releases free fatty acids, which travel to the already overworked liver to be stored and unfortunately contribute further to insulin resistance. In addition, central adipose tissue has been found to release inflammatory mediators, possibly contributing to the atherosclerotic process. This process is possibly monitored with one of the new markers for risk of CAD, the high-sensitivity C-reactive protein (hs-CRP or cardio-CRP).
| Waist over 40 inches |
| Triglycerides > 150 mg/dL |
| HDL cholesterol < 40 mg/dL |
| Hypertension (BP > 130/85) |
| Insulin resistance (fasting plasma glucose > 110 mg/dL) |
One method of quantifying insulin resistance is in the form of the hemoglobin A1C. From the EPIC-Norfolk Study, it was noted that for nondiabetic men, the difference between an A1C of 5.0 and 5.4 doubled their risk of a cardiovascular event.3 Perhaps these data explain why insulin-resistant men’s risk of a cardiovascular event increases 10 years before they become diabetic.
Regarding other risk factors, smoking is more common among men, and it is the most preventable risk factor for CAD. More than a quarter of men still smoke. Hypertension is slightly more common in men, with 26% being affected. Although men are less likely than women to be physically inactive, 30 to 50% are sedentary. Obesity and a sedentary lifestyle are directly associated with CAD in addition to contributing to most of the risk factors already mentioned.
Hypertension in Older Men
In the United States, hypertension is defined by the National Institutes of Health (in 1997) as systolic blood pressure (SBP) 140 mm Hg or greater, diastolic blood pressure (DBP) 90 mm Hg or greater, or taking antihypertensive medication. The World Health Organization (WHO) defines hypertension as a blood pressure higher than 160/95 mm Hg. Isolated systolic hypertension is defined as a SBP above 150 mm Hg with a DBP below 90 mm Hg. The estimates for the prevalence of hypertension in persons 65 years or older vary widely (10 to 50%) depending on the study and definition of hypertension used. Consistently, however, the prevalence of hypertension increases with age and the risk of cardiovascular events increases with the severity of the hypertension. In addition, the prevalence of hypertension in elderly black patients is higher than in elderly white patients. It is estimated that about one fifth of elderly men in the United States have isolated systolic hypertension (ISH).
The Framingham data has shown that ISH is more predictive for future stroke, myocardial infarction (MI), and congestive heart failure (CHF) than the presence of diastolic hypertension.4 Both reduced arterial compliance and increased cardiac output are implicated in the pathogenesis of ISH. Significant morbidity is associated with hypertension: the Framingham Heart Study showed a twofold increase in CHF in men in the presence of hypertension; in the Veterans Administration Cooperative Study on Antihypertensive Agents, half of all morbidity, heart failure, and stroke occurred in men older than 60 years even though they only made up 20% of the subjects in the trial. Fortunately, control of hypertension decreases the risk of cardiovascular death, heart failure, and stroke in elderly patients. Control of isolated systolic hypertension in the Systolic Hypertension in the Elderly Program (SHEP) using low-dose chlorthalidone as initial therapy and low-dose β-blockade added as needed reduced stroke by 36%, heart failure by 50%, and all cardiovascular events by 32%. Similar outcomes occurred in the Swedish Trial in Old Patients with Hypertension-2 (STOP-2) with therapies using β -blockade, diuretics, angiotensin-converting enzyme (ACE) inhibitors, and calcium channel blocking agents. Notably, elderly patients receiving ACE inhibitors had fewer MI and heart failure episodes compared with those patients treated with calcium channel blocking agents.
In elderly patients with mild hypertension, non-pharmacological therapies including reduction in salt intake, weight reduction, and regular physical activity should be considered. The goals of treatment should include lowering the blood pressure to 140/90mm Hg without causing postural hypotension, minimizing side effects, and using affordable drugs. More aggressive lowering of blood pressure should be considered in the hypertensive elderly patient who is also a diabetic or has chronic kidney disease. Initiating drug therapy at half the usual adult dose and measuring the blood pressure in the sitting and standing positions may minimize symptomatic side effects. Methods to promote long-term adherence should be considered, for example, providing written information describing the medical therapy and the blood pressure goal, and using simplified dosing regimens such as once-daily regimens, when possible, and pill-box systems.
Diagnosis of Heart Disease in Men
Compared with women, symptoms in men are much more specific for CAD. Typical angina is described as substernal chest discomfort relieved by rest or nitroglycerine. If the chest discomfort is characterized by two of these three factors, it is labeled atypical chest pain, and by one of the three factors, nonanginal chest pain. Tables and figures are available that delineate the likelihood of CAD by correlating these symptoms with age and gender. As it turns out, these tables alone are very accurate for predicting CAD in men. In fact, for men with typical angina, few studies need to be performed to confirm the diagnosis. From the tables, a 55-year-old man with typical angina has greater than a 90% chance of having CAD. In this case, a cardiac exercise test is not needed to make the diagnosis; an exercise test is usually more helpful for predicting the prognosis and managing the disease.
In addition, for men with a pretest likelihood of disease greater than 70%, the accuracy of the test is insufficient to exclude disease even if the test is negative. A positive exercise test is not much help in this range, such as in the previous example. An exercise test is also not useful in the lower pretest likelihood range (<20%) unless the test result is profoundly positive (>3mm ST segment deviation), which is rare. A patient with <20% pretest likelihood of disease with a positive exercise test is much more likely to have a false-positive test unless it is profoundly positive. Therefore, it may be safer to follow patients frequently until they develop a higher pretest likelihood of disease.
Fortunately, false-positive results are much less common for men undergoing exercise testing. In women, estrogen is apparently the cause of many false positives. With most experts now declaring only a flat or down-sloping ST-segment change as a positive, even false-positive results in women are minimized. The number of millimeters of ST-segment deviation should be measured at the j-point.
For those with a positive exercise test, the Duke nomogram is probably the most important development in the past 20 years for managing CAD. Use of the Duke nomogram allows the clinician to provide counseling that is very specific for the patient’s condition. In fact, patients can make informed decisions knowing their annual risk of a cardiovascular event if they choose medical as opposed to surgical management. In most centers, the risk of serious life-threatening complications from coronary artery bypass grafting (CABG) is ∼2.5%, so patients can compare their risk of complications with surgery against medical management or even watchful waiting.
When evaluating options for treatment, the exercise test is again helpful.5 There are study results that support medical management for men able to achieve 10 METS (metabolic equivalents of a task; 1 MET = amount of oxygen consumed at rest) with exercise, even if they have a positive result. However, this evidence needs to be considered in light of the age and activity level of the individual. For a man younger than 50 years of age, the diagnosis of CAD indicates aggressive CAD. It may be helpful to know the architecture of the coronary arteries; therefore, cardiac catheterization may be reasonable and helpful. For very active men older than 50 years, there may also be benefit of knowing the coronary architecture.
Because such a cardiac catheterization would be performed after having obtained objective evidence of ischemia (e.g., a positive exercise test or perfusion study), angioplasty or stenting may be reasonable at the time of the original catheterization. The current literature suggests that stenting may have surpassed angioplasty from the perspective of durability.
For those men with objective evidence of coronary ischemia at low work loads(<5 METS), revascularization is prudent, for which there are now options. CABG has been shown to improve outcomes of individuals with left main equivalent CAD (significant left main obstruction, or three-vessel obstruction with resultant left ventricular ischemic dysfunction). For all other men, angioplasty and/or stenting may be an option. The goal for most men undergoing angioplasty or stenting is to manage the symptoms or to buy time until their eventual CABG. Prior to our ability to aggressively manage cholesterol with medications, the average durability of a CABG was only 10 to 12 years. At that point, it usually had to be repeated. Therefore, the best candidate for a CABG is an elderly man, so that it will have to be performed only once or possibly twice. Durability data for angioplasty and stenting is still evolving and not fully available; therefore, the goal of these procedures is to buy time until the eventual CABG.
Primary Prevention
Primary prevention should be targeted toward risk factor reduction. Patients who smoke should be counseled to stop and should be enrolled in a smoking cessation program. Hypertension should be treated aggressively. Patients should be screened with a lipid profile at 21 years of age, and every 5 years thereafter if the profile is normal. For patients with multiple risk factors, or a strong family history of dyslipidemia, a lipid profile should be checked earlier. For those patients with multiple risk factors, it may also be prudent to measure a cardio-CRP level and possibly a homocysteine level. If homocysteine levels are elevated, there may be benefit of attempting to normalize them with high doses of folic acid (2–3 mg/day). Although we have few data proving the benefit of such treatment, the studies are currently ongoing and there is almost no detriment to folic acid supplementation. Folic acid is so safe that it was added to the commercially produced bread in the United States a few years ago.
Current evidence for medical management for the prevention of cardiovascular events indicates that aspirin is the most potent agent for prevention. As a primary preventive measure, 80 to 160 mg a day has been proven cardioprotective. The United States Preventive Task Force (USPSTF) recommends men take a low-dose aspirin daily starting at 40 years of age, and recently the AHA has recommended this dose beginning at the age of 50. For patients with multiple risk factors for CAD or an abnormal cardio-CRP, aspirin therapy should be started earlier.
For patients with multiple risk factors under the age of 50 years, there may be benefit of obtaining an electron beam computed tomography (EBCT) coronary calcium score. If the EBCT is abnormal, a cardiac exercise test should be performed. After the age of 50 years, a large percentage of patients have calcium on their EBCT so there is little value of an EBCT over a cardiac exercise test.
Interestingly, primary prevention goals in diabetics may be slightly different. And primary prevention is crucial in diabetics, because 8 out of 10 diabetics succumb to a cardiovascular event. From the United Kingdom Prospective Diabetes Study (UKPDS), more value was found in antihypertensives for preventing cardiovascular events than any other class of medications.6 Next most valuable were the lipid-lowering agents. Glycemic control also prevented events. Aspirin was also valuable for preventing cardiovascular events, but the number of patients needed to treat to prevent a cardiovascular event with aspirin was higher than with any of the other three classes of medications.
In nondiabetics, lipid-lowering agents clearly prevent cardiovascular events in high-risk individuals. Guidelines suggest treating anyone with greater than a 20% risk of a cardiovascular event over the next 10 years to obtain an LDL cholesterol level of less than 100 mg/dL. Lipid-lowering agents will also usually decrease cardio-CRP levels.
Patients should also be counseled to eat a low-fat, low-cholesterol diet or at least a Mediterranean-style diet. They should be given a prescription for exercise in the aerobic range at least 3 days a week for 30 minutes each day. Clinicians should spend some time learning about the topic of adherence to improve the likelihood that their patients will follow recommended treatments and lifestyle changes. Less than half of most medications are taken appropriately and even fewer lifestyle changes are maintained. As a result, the art and science of adherence are currently evolving.
Treatment and Secondary Prevention
As mentioned previously, for primary prevention clinicians must become experts in issues related to adherence if they want to be successful at treating and preventing events in patients with CAD. Adherence becomes even more of a challenge as the cost and number of medications increases and the lifestyle changes become more important. Such is the case in patients with CAD.
Antiplatelet therapy, including aspirin, is invaluable for secondary prevention following the initial diagnosis, a revascularization procedure, or a cardiovascular event. It may be the most effective medication available for preventing future cardiovascular events. Lifelong therapy in those individuals in whom it is not contraindicated is probably beneficial. For those in whom aspirin therapy is contraindicated, other antiplatelet agents should be considered, such as clopidogrel.
Lipid-lowering therapy is also indicated in most patients with CAD, with the ultimate goals still being determined. Most guidelines utilize LDL cholesterol as the treatment variable, and indicate a desirable LDL cholesterol goal is <100mg/dL in those patients with known CAD or cerebrovascular disease, or those with what is now determined to be a cardiovascular risk equivalent [e.g., diabetes, peripheral arterial disease (PAD), abdominal aortic aneurysm (AAA), symptomatic carotid disease]. Recent data from the Heart Protection Study and other studies, including several in progress, indicate that a goal for LDL cholesterol of < 100mg/dL may not be low enough. Lipid-lowering therapy not only prevents progression of disease but also has been shown to decrease cardiovascular events and mortality. It may even hasten regression of CAD.
For patients with impaired left ventricular function, ACE inhibitors are indicated. In addition, post-MI patients benefit from lifelong β-blocker therapy. Cardiac rehabilitation programs have also been proven to prevent cardiovascular events. Every necessary effort should be made to support the patient in an exercise program, including peripheral revascularization as needed. An exercise program will harm no patients with CAD, even those following an anterior MI, if they have been screened carefully by their clinician or through a cardiac rehabilitation program. In fact, most patients with known CAD should be advised to exercise for up to an hour a day, four or five times a week.
The patient should also be advised to follow a low-fat diet or a traditional Mediterranean diet. As it turns out, neither diet may affect the overall measured cholesterol levels; however, they have been shown to prevent cardiovascular events. Diets high in omega-3 fish oils have also been shown to prevent cardiovascular events.
Androgens and the Cardiovascular System
Heart disease is a very broad category of diseases, and this section addresses the impact of androgens on arteriosclerosis, blood pressure, and vascular disease. These three areas do have common etiological factors and are often interrelated. Hormones, including reproductive hormones, can regulate normal physiological factors and are discussed in more detail.
Sex steroids play a complex role in the vascular vessel wall system. In the past, many clinical trials have supported the protective effect of estrogens in arteriosclerosis. However, the recent Women’s Health Initiative estrogen replacement therapy failed to demonstrate a reduction of cardiovascular mortality.7 It seems that the effects of androgens on cardiovascular diseases is even more contentious, partly because of the lack of large clinical trials looking at this aspect of androgen influence in cardiovascular health. In the past, androgens were believed to be adverse to the cardiovascular system. However, recent studies in men have documented several beneficial actions of testosterone in the arterial vascular system. In short, androgens have been demonstrated to affect lipid metabolism including LDL, HDL, lipoprotein a [Lp(a)], and hemostasis through platelet aggregation and fibrinolytic activity. In addition, testosterone seems to affect coronary vessel dilatation. These effects are examined in the following subsections.
Arteriosclerosis and Androgens
In recent years there has been exciting evidence that androgens may indeed influence cardiovascular risk through the influence on arteriosclerosis. Some older men on androgen replacement seem to be protected from arteriosclerosis and hence arterial disease, but these trials are small and in no way conclusive. However, animal studies seem to suggest the protection of some species but not all species from arteriosclerosis.
One of the paradoxes of androgens on cardiovascular health is that they tend to reduce HDL, and HDL has been viewed to be cardioprotective. The reduction of HDL and elevation of LDL is not universal in all cases of androgen supplementation, and the issue is less important in older men.8 On the positive side, the administration of androgens also results in a reduction of triglyceride and Lp(a), which in turn are also independent risk factors for arteriosclerosis.9 Androgen supplementation can also increase the hematocrit, and in some older men with heart failure this could be seen as a positive effect. Increases in the hematocrit can result in relief of symptoms of shortness of breath seen in advanced cases of heart failure. Quality of life may thus be enhanced with androgen supplementation in selected patients.
One of the larger studies to determine the association between hypotestosteronemia and arteriosclerosis has been the population-based Rotterdam study (Fig. 13–1),10 which investigated the association of levels of dehydroepiandrosterone sulfate (DHEAS) and total and bioavailable testosterone with aortic arteriosclerosis among 1032 nonsmoking men and women over 55 years. Aortic arteriosclerosis was assessed by radiographic detection of calcified deposits in the abdominal aorta. Relative to men with levels of total and bioavailable testosterone in the lowest tertile, men with levels of these hormones in the highest tertile had age-adjusted relative risks of 0.4 [95% confidence interval (CI), 0.2–0.9] and 0.2 (CI, 0.1–0.7), respectively, for the presence of severe aortic arteriosclerosis. The corresponding relative risks for women were 3.7 (CI, 1.2–11.6) and 2.3 (CI, 0.7–7.8). In this longitudinal study, men with levels of total and bioavailable testosterone in subsequent tertiles were also protected against progression of aortic arteriosclerosis measured after 6.5 year (SD/ ±0.5 year) of follow-up (p for trend = .02). No clear association between levels of DHEAS and presence of severe aortic arteriosclerosis was found, either in men or in women. In men, a protective effect of higher levels of DHEAS against progression of aortic arteriosclerosis was suggested, but the corresponding test for trend did not reach statistical significance.
This study also found an independent inverse association between levels of testosterone and aortic arteriosclerosis in men. In women, however, positive associations between levels of testosterone and aortic arteriosclerosis were largely due to adverse cardiovascular disease risk factors. Epidemiological studies point to a protective effect of endogenous testosterone against arteriosclerosis. It is instinctive to suggest that administration of testosterone to older men would protect against arteriosclerosis, but long-term prospective trials looking at this issue are not yet available. Fig. 13–2 shows increasing risk of arteriosclerosis with decreasing quartile of testosterone in men but not women.
Blood Clotting and Androgens
As seen in the previous discussion, hypotestosteronemia is associated with an increased risk of cardiovascular disease. In the literature, there is some evidence that low baseline fibrinolytic activity in hypogonadal men results in thromboembolic disease and perhaps even myocardial infarction. We know from studies that hypogonadism in men is associated with an enhancement of fibrinolytic inhibition via increased synthesis of the plasminogen activator inhibitor-1 (PAI-1).11
It has been demonstrated that synthetic androgens such as stanozolol and danazol reduce PAI-1 and are associated with increased fibrinolytic activity.12 However, in men who abuse anabolic steroids and who typically use supraphysiological levels of androgens, thrombosis has been reported.13 A prothrombotic state appears when the threshold dose is exceeded. There have been numerous reports on weightlifters dying of atherothrombotic and ischemic heart disease while abusing anabolic steroids.
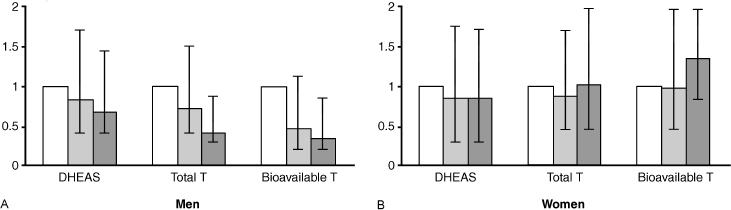
FIGURE 13-1. Results of the population-based Rotterdam study for (A) men and (B) women to determine the association between DHEAS, total testosterone, and bioavailable testosterone and severe aortic arteriosclerosis in 1032 nonsmoking men and women above the age of 55. There was a decrease in severe aortic arteriosclerosis with increases in total and bioavailable testosterone (T). No statistical effect of increases in DHEAS was found. (Adapted from Hak AE, Witteman JC, de Jong FH, et al. Low levels of endogenous androgens increase the risk of atherosclerosis in elderly men: the Rotterdam study. J Clin Endocrinol Metab 2002;87(8):3632–3639, with permission.)

FIGURE 13-2. Effect of free testosterone (T) on the degree of coronary artery disease in a group of patients. Free testosterone correlated with a decrease in degree of coronary artery disease. (Adapted from Phillips GB, Pinkernell BH, Jing TY. The association of hypotestosteronemia with coronary artery disease in men. Arterioscler Thromb 1994; 14:701–706, with permission.)
Besides the activity of androgens on blood clotting, there are also concomitant androgen effects on carbohydrate and lipid metabolism. Some of the individual inconsistency of the effects of androgens on fibrinolytic and hemostatic activity appears to be based on the interrelationship to these metabolic systems. Androgens may have unfavorable effects on the HDL/LDL cholesterol ratio, but may have favorable effects on triglycerides and insulin resistance. Hypertriglyceridemia and insulin resistance are both associated with low fibrinolytic activity and increased PAI-1 levels, and hence androgens may influence clotting through indirect means.
Lipoprotein(a), a recently acknowledged independent risk factor of cardiovascular disease (CVD), has been shown to respond favorably to androgen treatment in not only men but also women. A synthetic androgen medication such as danazol used in premenopausal women for endometriosis was found to reduce PAI-1, suggesting an improvement of the fibrinolytic activity.14 In addition, hormone replacement therapy (HRT) in women with androgenic progestins are associated with a marked reduction of PAI-1 and thus an improvement of fibrinolytic activity. Further improvement of fibrinolytic activity may be associated with the marked decrease of lipoprotein(a) in women who are on HRT with androgens in combination.
In summary, the current information on the effects of androgens on blood clotting is limited. Part of the difficulty is due to the interaction of androgens with other sex steroids in influencing blood clotting. Synthetic androgens have been shown to influence the synthesis and release of hemostatic factors. These include an increase of the inhibitors of coagulation and a decrease of the inhibitor of the fibrinolytic system. However, the use of androgens in patients with congenital deficiencies of these coagulation factors or previous events of cardiovascular disease has yielded disappointing results.12 It is with optimism that androgen effects on the reduction of fibrinolytic inhibition (PAI-1) and Lp(a) can lead to the reduction of risk of cardiovascular disease. The profibrinolytic effects of androgens may be of particular interest, and clinical trials are needed in men to determine if clinical outcomes of increased thrombotic states like strokes and myocardial infarctions can indeed be reduced with bioidentical androgen replacement.
Blood Pressure and Androgens
It seems that gender may play an important role in blood pressure. For instance, premenopausal women have lower arterial blood pressure than age-matched men. Postmenopausal women have higher blood pressures, however, suggesting that ovarian hormones can possibly alter blood pressure. Again, it is intuitive to suggest that androgens may be responsible for higher blood pressure in men as much higher levels of androgens are found in men. However, with aging, hypotestosteronemia results and older men have higher blood pressures, which suggests that testosterone may protect against rises in blood pressure. There is a danger of overinterpreting these associations, which may not be causal relationships.
For instance, animals administered testosterone have shown induction of hypertension and hypertrophy of the left ventricle. Crofton and Share15 demonstrated that in rats with deoxycorticosterone (DOC)-salt hypertension, arterial blood pressure rises more rapidly and reaches a higher level in male than in female rats. However, the course of the hypertension was removed by gonadectomy in male rats but exacerbated by gonadectomy in female rats. The group also found that testosterone aggravates the development of the hypertension in gonadectomized male rats but not in intact females. Progesterone, which has androgenic effects, had no effect on hypertension in ovariectomized rats but when given to ovariectomized rats in combination with estradiol transiently prevented the protective effect of the estradiol. The findings suggest that gonadal steroid hormones may play an important role in modulating the pathogenesis of DOC-salt hypertension in rats. It was suggested that the effects of the gonadal hormones on the course of the hypertension might be due to modulation of the cardiovascular and renal actions of vasopressin, because vasopressin is required for this model of hypertension.
In contrast, the effect of testosterone on blood pressure in humans has been positive rather than negative as described previous in animal models. For example, in the Rancho Bernardo study in San Diego, there was a negative correlation between plasma testosterone and testosterone levels (Table 13–2).16
In that study of 1132 men aged 30 to 79 years, those with hypertension, categorically defined as SBP greater than 160 mm Hg and/or DBP greater than 95mm Hg had significantly lower testosterone levels than non-hypertensives. SBP and DBP are inversely correlated with testosterone levels (r = 0.17, p < .001 for systolic; r = 0.15, p < .001 for diastolic) in the whole cohort. The association was present over the whole range of blood pressures and sex hormone levels, with a stepwise decrease in mean SBP and DBP per increasing quartile of testosterone. Obesity accounted for some, but not all, of this relationship, which was reduced, but still apparent after adjusting for age and body mass index. It was of interest that no other hormone (androstenedione, estrone, estradiol) or even sex hormone–binding globulin showed a consistent relationship with blood pressure. However, the association does not prove causality.
To take this association further to prove causality, exogenous testosterone can be administered to men and their blood pressure can then be observed. However, these intervention trials generally use small numbers of subjects, and thus it is difficult to come to a consensus. In three separate small studies, investigators found no negative influences on blood pressure either with physiological or supraphysiological levels of exogenous testosterone.17–19 In contrast to testosterone, however, anabolic steroids used for bodybuilding may cause a slight rise in blood pressure. In one such study, the effects of anabolic steroids on body composition, blood pressure, lipid profile, and liver functions were studied in male body builders who received a weekly intramuscular injection of nandrolone-decanoate (100 mg) or placebo for 8 weeks in a double-blind study.20 An increase in diastolic blood pressure was observed, which returned to pre-anabolic values ∼6 weeks after cessation of drug administration.
In conclusion, animal models suggest that testosterone administration leads to a rise in blood pressure. However, in human observations, testosterone appears to behave as a coronary and possibly peripheral vasodilator, acting primarily through nitric oxide release and the modulation of endothelial function.21 Testosterone introduced into the coronary arteries during angiography results in a dose-dependent increase in vessel diameter and blood flow.22 The administration of physiological doses of testosterone to older men who are hypogonadal appears not to influence blood pressures adversely, and may possibly even be beneficial. As explained, the use of anabolic steroids for bodybuilding is associated with a slight rise in blood pressure, which in turn is reversible. The effect of DHEA and androstenedione on blood pressure is unclear at this point.
Influence of Androgens on Coronary and Peripheral Circulation
Testosterone had been suspected of being a coronary vasodilator as far back as the 1940s. Four separate trials in that decade demonstrated the relief of angina pectoris with testosterone injections.23–26 Several cross-sectional studies also suggest a positive correlation of hypotestosteronemia to poor arterial circulation. For instance, Phillips and his group27 demonstrated that testosterone correlated negatively with the risk factors fibrinogen, PAI-1, and insulin, and positively with high-density lipoprotein cholesterol. The correlations found in their study between testosterone and the degree of coronary artery disease and between testosterone and other risk factors for MI raise the possibility that in men hypotestosteronemia may be a risk factor for coronary arteriosclerosis (Fig. 13–2).
Models of androgen deprivation in men, as seen in chemical castration for prostate cancer, has demonstrated that androgen withdrawal is associated with a reduction in central arterial compliance.28 Arterial compliance or “stiffness” is increasingly regarded as a modifiable risk factor for cardiovascular disease. In a study by Webb and her colleagues,22 the hypothesis that testosterone has direct relaxing effects on coronary arteries was furthered. Thirteen older men with coronary artery disease underwent measurement of coronary artery diameter and blood flow after a 3-minute intracoronary infusion of vehicle control (ethanol) followed by 2-minute intracoronary infusions of acetylcholine. A dose-response curve to 3-minute infusions of testosterone was then determined, and the acetylcholine infusions were repeated. Finally, an intracoronary bolus of isosorbide dinitrate was given. Coronary blood flow was calculated from measurements of blood flow velocity, using intracoronary Doppler, and coronary artery diameter, using quantitative coronary angiography Testosterone significantly increased coronary artery diameter compared with baseline. A significant increase in coronary blood flow also occurred at all concentrations of testosterone compared with baseline. Fig. 13–3 shows the effect of testosterone on coronary diameter and blood flow.
Unfortunately, the Cochrane systematic review of testosterone replacement research concluded that there is no evidence to date that short-term testosterone treatment is beneficial in subjects with lower limb arteriosclerosis. However, the authors admitted that their conclusion was based on limited data rather than the lack of a real effect.29 The clinical significance of the collective data are that because testosterone may act as a vasodilator, hypogonal patients on nitrate therapy may attain better coronary blood flow if they are replaced to physiological levels of testosterone. Testosterone should not replace conventional therapies for coronary artery disease including nitrates, calcium channel blockers, (3-blockers, and others, but could be considered as an adjunct in difficult selected cases if the patient is hypogonadal and there are no contraindications.
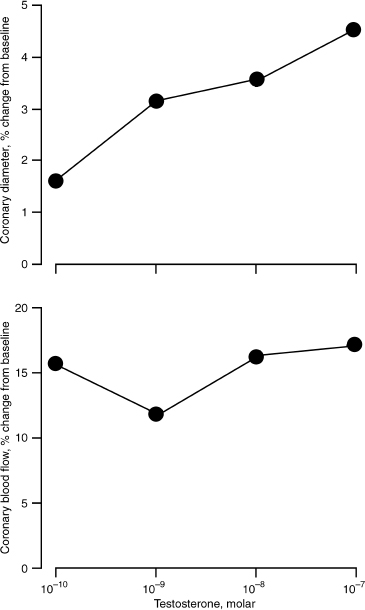
FIGURE 13-3. Effect of testosterone on coronary artery diameter and blood flow. Testosterone correlated significantly with increased coronary artery diameter and blood flow at all levels of administration. (Adapted from Webb C, McNeill J, Hayward C, et al. Effects of testosterone on coronary vasomotor regulation in men with coronary heart disease. Circulation 1998;100:1960–1966, with permission.)
Discussion of the Case History
The patient immediately reported improved quality of life after being on testosterone. Partly because of his increased energy from the testosterone, he began to exercise more regularly. Subsequent follow-ups reveal that he lost 40 pounds in total after a year of supervision. His blood pressure went down to 100/80 mm Hg without adjusting his hypertension medications. Felodipine was discontinued, and his pressure normalized to 120/80 mm Hg. The drop in blood pressure is a reflection of his weight loss, increased aerobic activity, and possibly the vasodilatory effects of testosterone. His chest pains totally disappeared. His cholesterol levels did not alter much, and he was maintained on the same dose of atorvastatin.
Conclusion and Key Points
Much has been studied about estrogens and heart disease in women. Androgens certainly play a role in the cardiovascular well-being of men. Trials of testosterone therapy in men are limited, and much more research needs to be done before the effects of testosterone therapy are fully understood. However, the impact of androgens on the cardiovascular system is generally positive, rather than negative, especially when physiological doses are used. Supraphysiological doses as administered by some athletes can be harmful.
• There is still no consensus of the impact of androgens on arteriosclerosis.
• However, it has been demonstrated that androgen therapy decreases fibrinogen, lipoprotein, and PAI-1, all of which are risk factors for arteriosclerosis.
• Hypotestosteronemia in itself is associated with coronary arteriosclerosis.
• Hypotestosteronemia in itself is also associated with hypertension.
• Testosterone replacement does not induce hypertension, but may even be useful in lowering pressure.
• There is increasing evidence that physiological, but not supraphysiological, doses of testosterone result in vasodilatation of coronary and peripheral vasculature.
• Angina is not worsened by testosterone, but may even be improved with it.
REFERENCES
1. McGill HC Jr, Mcmahan CA, Zieske AW, et al. Association of coronary heart disease risk factors with the intermediate lesion of atherosclerosis in youth: the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Arte-rioscler Thromb Vasc Biol 2000;20:1998–2004
2. Berenson GS, Wattigney WA, Tracey RE, et al. Atherosclerosis of the aorta and coronary arteries and cardiovascular risk factors in persons aged 6 to 30 years and studied at necropsy: the Bogalusa Heart Study. Am J Cardiol 1992;70:851–858
3. Khaw KT, Wareham N, Luben R, et al. Glycated hemoglobin, diabetes, and mortality in men in Norfolk cohort of European prospective investigation of cancer and nutrition (EPIC-Norfolk). BMJ 2001;322:15–18
4. Witteman JC, D’Agostino RB, Stijnen T, et al. G-estimation of causal effects: isolated systolic hypertension and cardiovascular death in the Framingham Heart Study. Am J Epidemiol 1998;148:390–401
5. Fowler GC, Evans CH, Altman MA. Office procedures: exercise testing. Prim Care 1997;24:375–406
6. Stratton IM, Adler AI, Neil HA, et al. Association of glycemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405–412
7. Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA 2002;288:321–333
8. Bhasin S, Buckwalter JG. Testosterone supplementation in older men: a rational idea whose time has not yet come. J Androl 2001;22:718–731
9. Barud W, Palusinski R, Beltowski J, Wojcicka G. Inverse relationship between total testosterone and anti-oxidized low-density lipoprotein antibody levels in ageing males. Atherosclerosis 2002;164:283–288
10. Hak AE, Witteman JC, de Jong FH, et al. Low levels of endogenous androgens increase the risk of atherosclerosis in elderly men: the Rotterdam study. J Clin Endocrinol Metab 2002;87:3632–3639
11. Zollner TM, Veraart JC, Wolter M, et al. Leg ulcers in Klinefelter’s syndrome–further evidence for an involvement of plasminogen activator inhibitor-1. Br J Dermatol 1997;136:341–344
12. Winkler UH. Effects of androgens on haemostasis. Maturitas 1996;24:147–155
13. Ferenchick GS, Hirokawa S, Mammen EF, et al. Anabolic-androgenic steroid abuse in weight lifters: evidence for activation of the hemostatic system. Am J Hematol 1995;49: 282–288
14. Ledford MR, Horton A, Wang G, et al. Efficacy of danazol in a patient with congenital protein-S deficiency: paradoxical evidence for decreased platelet activation with increased thrombin generation. Thromb Res 1997;87:473–482
15. Crofton JT, Share L. Gonadal hormones modulate deoxy-corticosterone-salt hypertension in male and female rats. Hypertension 1997;29:494–499
16. Khaw KT, Barrett-Connor E. Blood pressure and endogenous testosterone in men: an inverse relationship. J Hypertens 1988; 6:329–332
17. Whitworth JA, Scoggins BA, Andrews J, et al. Haemodynamic and metabolic effects of short term administration of synthetic sex steroids in humans. Clin Exp Hypertens A 1992;14:905–922
18. White CM, Ferraro-Borgida MJ, Moyna NM, et al. The effect of pharmacokinetically guided acute intravenous testosterone administration on electrocardiographic and blood pressure variables. J Clin Pharmacol 1999;39:1038–1043
19. Marin P, Holmang S, Jonsson L, et al. The effects of testosterone treatment on body composition and metabolism in middle-aged obese men. Int J Obes Relat Metab Disord 1992;16:991–997
20. Kuipers H, Wijnen JA, Hartgens F, et al. Influence of anabolic steroids on body composition, blood pressure, lipid profile and liver functions in body builders. Int J Sports Med 1991; 12:413–418
21. Lloyd G. Androgens and blood pressure in men. In: Textbook of Men’s Health. London: Parthenon; 2002:351–359
22. Webb C, McNeill J, Hayward C, et al. Effects of testosterone on coronary vasomotor regulation in men with coronary heart disease. Circulation 1999;100:1690–1696
23. Hamm L. Testosterone propionate in the treatment of angina pectoris. J Clin Endocrinol 1942;2:325–328
24. Siegler LH, Tuglan J. Treatment of angina pectoris by testosterone propionate. NY State J Med 1943;43:1424–1428
25. Walker TC. The use of testosterone propionate and estrogenic substance in the treatment of essential hypertension, angina pectoris and peripheral vascular disease. J Clin Endocrinol 1942;2:560–568
26. Lesser MA. Testosterone propionate therapy in one hundred cases of angina pectoris. J Clin Endocrinol 1946; 6:547–549
27. Phillips GB, Pinkernell BH, Jing TY. The association of hypotestosteronemia with coronary artery disease in men. Arte-rioscler Thromb 1994;14:701–706
28. Dockery F, Bulpitt CJ, Agarwal S, et al. Testosterone suppression in men with prostate cancer is associated with increased arterial stiffness. Aging Male 2002;5:216–222
29. Price JF, Leng GC. Steroid sex hormones for lower limb arteriosclerosis. Cochrane Database Syst Rev 2002;1:CD000188
< div class='tao-gold-member'>










