Peter Stenvinkel, Charles A. Herzog
Cardiovascular Disease in Chronic Kidney Disease
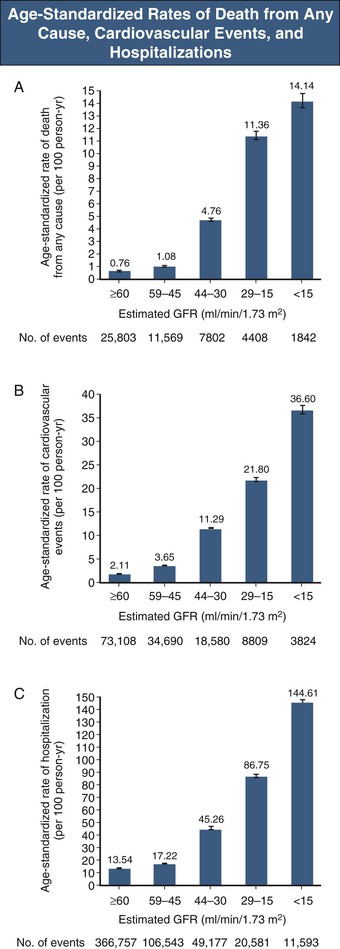
The life span of patients with chronic kidney disease (CKD), particularly those with end-stage renal disease (ESRD), is reduced. In the United States, the all-cause mortality rate of prevalent dialysis patients in 2010 was 193 deaths per 1000 patient-years, 38% attributable to cardiac causes.1 Diminished estimated glomerular filtration rate (eGFR) is a powerful graded, independent predictor of cardiovascular morbidity and mortality2 and all-cause mortality (Fig. 82-1).3 Even subtle kidney dysfunction, as suggested by albuminuria, increases cardiovascular risk4 because it may reflect microvasculature health, including endothelial function. There is a strong association between urinary albumin excretion and other cardiovascular risk factors.5 ESRD patients face an extraordinary risk for premature death, largely as a result of cardiovascular complications. However, the numbers of patients with non–dialysis-dependent CKD are much larger, and those with eGFR below 60 ml/min/1.73 m2 are much more likely to die than to develop ESRD,6 reflecting the burden of cardiovascular disease (CVD) in this high-risk population. The most effective strategy for reducing cardiovascular morbidity and mortality would be to target patients with mild renal impairment for prevention and treatment before severe CKD develops.
Unfortunately, CKD patients were often excluded from randomized controlled trials targeting CVD, or renal function was poorly described,7 possibly reducing acceptance of evidence-based therapies (validated in nonrenal patients) and fostering “therapeutic nihilism” in clinicians who treat CKD patients.
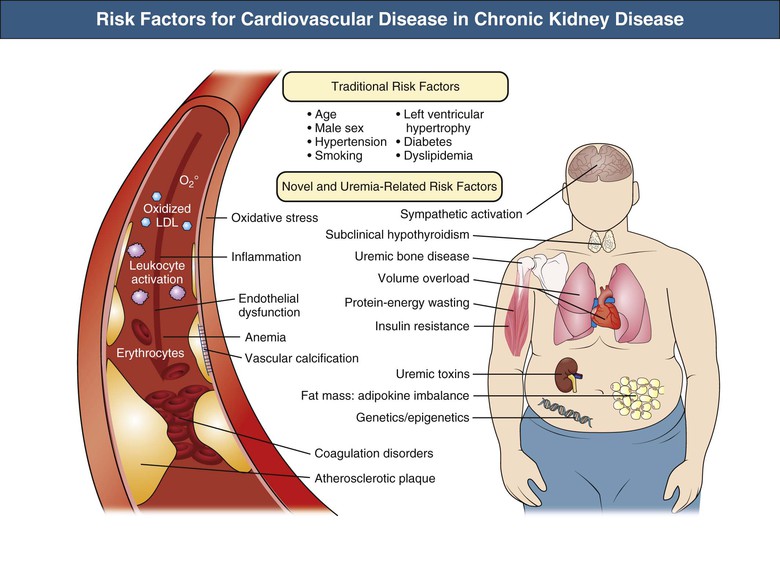
Like conventional atheromatous occlusive vascular disease, CKD is characterized by generalized vasculopathy with other characteristics, including left ventricular hypertrophy (LVH), vascular calcification, and vascular noncompliance. Numerous CVD risk factors are specific for CKD and operate in addition to conventional risk factors found in the general population.
Epidemiology
Prevalence of Cardiovascular Complications in Chronic Kidney Disease
Interpretation of epidemiologic studies of CVD in CKD is problematic because of the difficulty in defining cause of death, but this difficulty is not limited to renal disease patients. Unexpected sudden death is most likely a result of arrhythmia, but a subarachnoid hemorrhage, massive embolic stroke, or aortic dissection might be indistinguishable from a primary arrhythmic event without an autopsy. The real conundrum, however, is defining “coronary heart disease” (CHD). In the general population, sudden cardiac death is rightly considered a primary complication of CHD; evidence-based interventions (e.g., statins) aimed at quelling the progression of atherosclerotic disease reduce the incidence of sudden death. However, in dialysis patients, sudden cardiac death is likely not to be a surrogate for CHD. Even use of a history of angina to classify a patient as having CHD is problematic because angina (as a result of supply-demand mismatch) can occur in patients with LVH and angiographically pristine coronary arteries. This probably relates to the increased myocardial fibrosis, diminished relative capillary density, and increased thickening of the intramyocardial vessel walls in uremia.
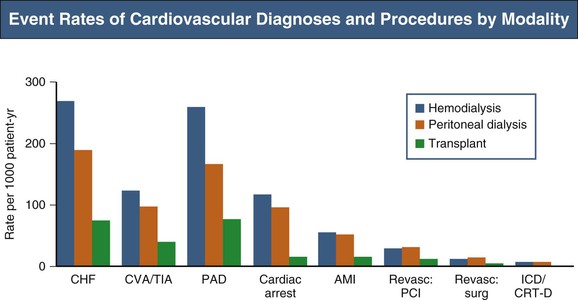
Of incident ESRD patients, 75% have LVH. Hypertension prevalence increases progressively with falling GFR; 75% to 85% of dialysis patients have hypertension. With hypertension, anemia, vascular noncompliance, and volume overload contribute to LVH. Based on echocardiography, 85% to 90% of patients have a left ventricular (LV) ejection fraction (EF) of 50% or higher, despite frequent congestive heart failure (CHF).8 Because CHF is diagnosed in about 40% of incident ESRD patients within the first year,9 many volume overload episodes may be attributable to diastolic dysfunction or circulatory congestion. CHF hospitalizations are associated with high long-term mortality.10 Although occlusive CHD is common in CKD patients, acute myocardial infarction (AMI) accounts for only 13% of cardiac deaths; 69% of deaths in the United States Renal Data System (USRDS) database are attributable to arrhythmias.11 In incident elderly dialysis patients, new atrial fibrillation (AF) occurs at an annual rate of 15%, with 59% mortality in the first year after incident AF.12 Figure 82-2 provides a snapshot of CVD event rates in prevalent ESRD patients.
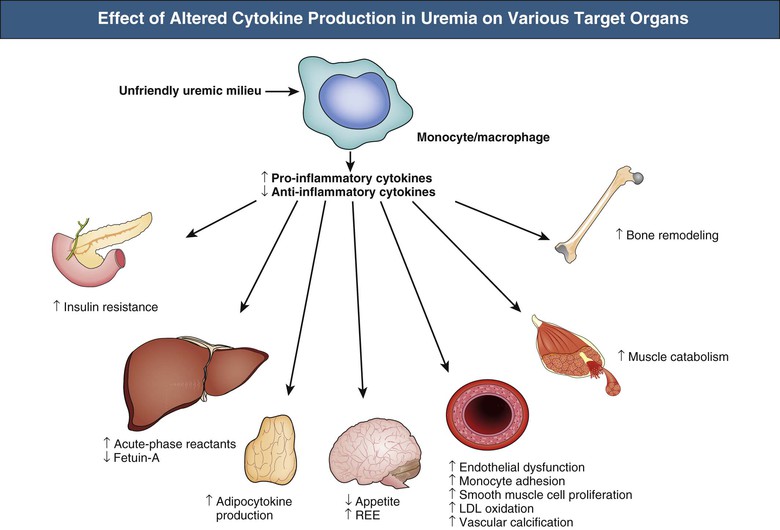
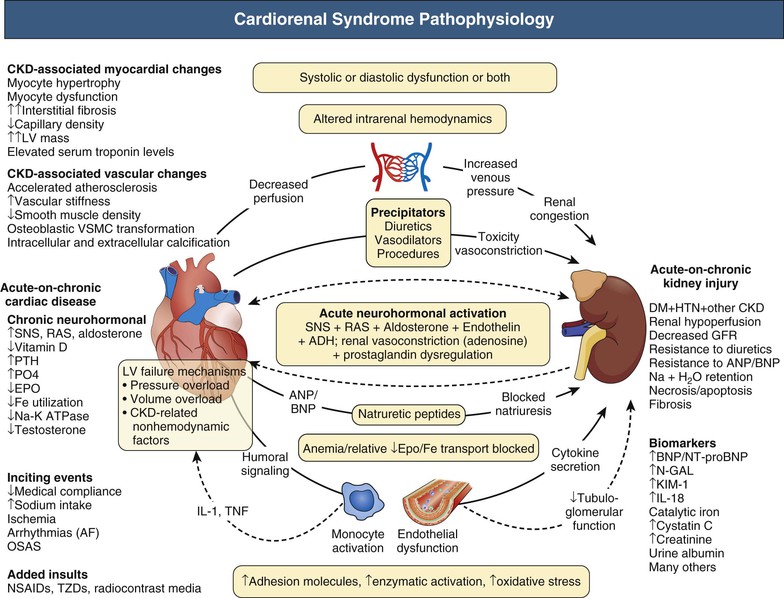
Congestive heart failure results from pressure overload, volume overload, and nonhemodynamic factors altering the myocardium.13 Figure 82-3 summarizes cardiorenal syndrome pathophysiology leading to cardiomyopathy and CHF.
Cardiovascular Disease Is Present Before the Start of Renal Replacement Therapy
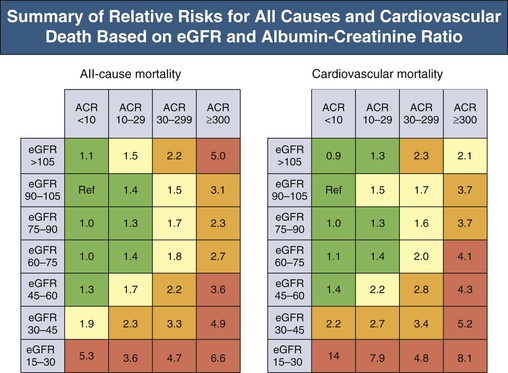
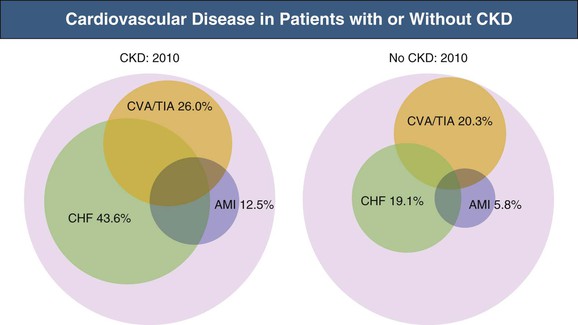
In elderly CKD patients at stage 2 or 3, traditional risk factors seem to be the major contributors to cardiovascular mortality. Atherosclerosis Risk in Communities (ARIC) data suggest that both traditional and novel risk factors are relevant at CKD stage 4,14 and studies of dialysis patients suggest that novel risk factors are far more prevalent than in the general population (Fig. 82-4).15 The Framingham predictive instrument does not accurately predict coronary events in CKD.16 Mild to moderate CKD is associated with increased risk of venous thromboembolism,17 supporting the concept of hypercoagulability in CKD. A Kidney Disease: Improving Global Outcomes (KDIGO) collaborative meta-analysis provides estimates of cardiovascular and all-cause mortality risk stratified by eGFR and urinary albumin-creatinine ratio (Fig. 82-5).18 Figure 82-6 shows the burden of CVD in elderly patients with and without non–dialysis-dependent CKD.
Racial and International Differences in Cardiovascular Disease Prevalence
In the United States, African American dialysis patients have better survival than Caucasian dialysis patients. However, overall cardiovascular mortality among dialysis patients from the United States is significantly greater than is observed in Japan and Europe,19 even after adjustment for standard risk factors and dialysis dose. Higher mortality rates in U.S. dialysis patients may be related to higher prevalence of sicker or diabetic patients or to differences in dialysis practice patterns; however, cultural habits, differences in diet, or genetic variations may also contribute.
Reverse Epidemiology
Reverse epidemiology refers to the paradoxical observation that the association among hypercholesterolemia, hypertension, obesity, and poor outcomes, including cardiovascular death, in the general population does not exist and may be reversed in the CKD population.20 This concept is needlessly confusing and should be replaced by confounded epidemiology. Causality must not be confused with association. It is assumed (but open for debate) that patients with wasting and inflammation mostly account for poor survival and confounded epidemiology.
Etiology and Risk Factors
Traditional Risk Factors
Age, Gender, and Smoking
The U.S. National Health and Nutrition Examination Surveys (NHANES) show the prevalence of cardiovascular risk factors and CVD prevalence in relation to age and CKD stage. In the United States, the average age at renal replacement therapy initiation is 63 years, when CVD is common. An individual-level meta-analysis including more than 2 million participants showed that low eGFR and high albuminuria were independently associated with mortality and ESRD regardless of age.21 Female gender is associated with a 4% independent increased risk of mortality in incident dialysis patients,5 and smoking with a 52% increased risk of death in dialysis patients.22
Diabetes Mellitus
Diabetes accounted for 44% of incident U.S. ESRD patients in 20101 and is the most common cause of ESRD in many countries. Diabetic patients starting renal replacement therapy have numerous cardiovascular risk factors, including dyslipidemia, hypertension, persistent inflammation, increased oxidative stress, and protein-energy wasting. Not surprisingly, diabetes at dialysis initiation is an independent risk factor for all-cause and CVD-related deaths, including after coronary revascularization or AMI; diabetic ESRD confers a 34% mortality risk after AMI compared with nondiabetic ESRD. Despite diabetes being a “CHD equivalent,” the rate of incident AMI is even higher for patients with CKD stages 3b to 4 without diabetes than for patients with diabetes and eGFR of 60 ml/min/1.73 m2 or higher.23
Hypertension
Hypertension is common but variably treated in CKD patients. Of NHANES5 participants with CKD stages 3 and 4, 80% were hypertensive (defined as blood pressure ≥130/≥80 mm Hg for CKD patients), and only 20% were aware of it and treated, with the blood pressure adequately controlled. Of participants with CKD stages 1 and 2, 63% were hypertensive and only 11% of these were treated, with blood pressure adequately controlled. More recent data from NHANES 2001 to 2010 suggest modest improvement in hypertensive people with CKD stages 3 to 4.24 Low blood pressure is correlated with mortality in some dialysis patients (see the discussion of reverse epidemiology). However, as in the general population, hypertension predicts mortality in CKD patients before or at dialysis initiation. Isolated systolic hypertension with increased pulse pressure is by far the most prevalent blood pressure anomaly in dialysis patients, resulting from arterial medial sclerosis with secondary stiffening. Stiff vessels cause increased pulse wave velocity, resulting in increased systolic peak pressure by a prematurely reflected pulse wave, progressive LV dysfunction, and finally CHF. This may subsequently result in decreased mean arterial and diastolic pressure and increased risk for cardiovascular death. The relationship between blood pressure and mortality is U shaped; isolated systolic hypertension and increased pulse pressure probably indicate high long-term risk in dialysis patients, whereas low mean and diastolic blood pressures predict early mortality. The invisible hypertension danger relates to numerous CKD patients being “nondippers” (see Chapter 33). CKD patients frequently also experience sleep apnea associated with nondipping, sympathetic nervous system activation, and cardiovascular risk.
Dyslipidemia
The relationship among hypercholesterolemia, CVD, and mortality in CKD patients is weak because some major cardiovascular abnormalities, such as cardiomyopathy and arteriosclerosis, may be less dependent on dyslipidemia than on other factors. Paradoxically, low rather than high serum cholesterol level is associated with poor survival in hemodialysis (HD) patients,20 confounded epidemiology likely related to protein-energy wasting and inflammation. After adjustment for C-reactive protein (CRP) levels, high cholesterol level predicted risk in relatively healthy ESRD patients without inflammation.25
Progressive CKD leads to changes in blood lipids associated with vascular disease, including decreased levels of apolipoprotein A (apoA)–containing lipoproteins and increased levels of apoB-containing lipoproteins (Table 82-1). Plasma triglyceride levels are elevated in most ESRD patients, whereas total serum cholesterol levels may be elevated, normal, or low, depending on nutritional status and presence of inflammation. High-density lipoprotein (HDL) cholesterol is typically reduced, and low-density lipoprotein (LDL), intermediate-density lipoprotein, and very low-density lipoprotein cholesterol as well as lipoprotein(a) levels tend to be increased. Because of specific changes in its molecular composition, HDL anti-inflammatory activities are defective in the uremic milieu.26 Compared with long-term HD patients, peritoneal dialysis (PD) patients more often have both hypercholesterolemia and hypertriglyceridemia. Both groups are characterized by low HDL and elevated oxidized LDL cholesterol levels; elevated lipoprotein(a) levels are associated with increased CVD mortality.
Table 82-1
Lipid abnormalities in renal disease.
Common patterns of hyperlipidemia in different stages of renal disease, compared with the healthy population.
| Lipid Abnormalities in Renal Disease | ||||
| Cholesterol Levels | ||||
| Stage of Renal Disease | Total | High-Density Lipoproteins | Low-Density Lipoproteins | Triglycerides |
| Nephrotic syndrome | ↑ ↑ ↑ | ↓ | ↑ ↑ | ↑ |
| Chronic kidney disease | No change | ↓ | No change* | ↑ ↑ |
| Hemodialysis | No change | ↓ | No change* | ↑ ↑ |
| Peritoneal dialysis | ↑ | ↓ | ↑ | ↑ |
| Transplantation | ↑ ↑ | No change | ↑ | ↑ |
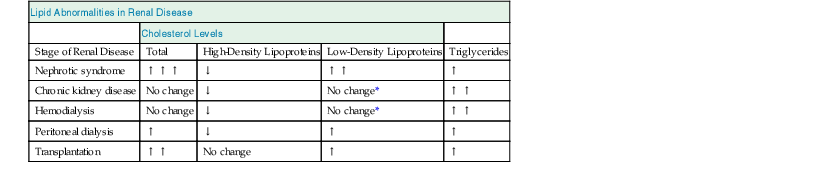
* Composition altered.
Insulin Resistance and Atherosclerosis
In the general population, impaired insulin-stimulated glucose disposal in muscle is often part of a metabolic syndrome that includes dyslipidemia, hypertension, endothelial dysfunction, and sympathetic overactivity. Many of these abnormalities are present in CKD. Although insulin resistance was found to be an independent predictor of cardiovascular mortality in dialysis patients,27 its contribution to CKD patient mortality is uncertain.
Nontraditional and Uremia-Specific Risk Factors
Several large prospective population studies have shown that even mild CKD is an independent risk factor for CVD, independent of hypertension, diabetes, and albuminuria, and similar in magnitude to diabetes and hypertension.28,29 The uremic milieu may affect both quality and quantity of the atherosclerotic plaques. Coronary lesions in uremic patients, compared with nonrenal controls, are characterized by increased media thickness, infiltration, and activation of macrophages and marked calcification.30 The mechanism by which a uremic milieu may accelerate atherosclerosis is not well established, but prevalence and magnitude of several nontraditional risk factors, such as oxidative stress, inflammation, vascular calcification, and advanced glycation end products (AGEs), increase as renal function deteriorates. Other uremic retention solutes, such as asymmetric dimethylarginine (ADMA), guanidine, indoxyl sulfate, and p-cresol, which accumulate in CKD, may have proatherogenic properties.31 Finally, the kidneys produce substances that may inhibit CVD and atherogenesis—for example, renalase, a soluble monoamine oxidase that regulates cardiac function and blood pressure.32
Oxidative Stress
Oxidative stress may be implicated in the pathogenesis of atherosclerosis and the increased risk of atherosclerotic cardiovascular events and in other CKD complications, such as protein-energy wasting and anemia.33 Increased production of reactive oxygen species in the vascular wall is a characteristic feature of atherosclerosis.33 Early CKD (stage 3), and in particular uremia, is a pro-oxidant state resulting from reduced antioxidant systems (vitamin C and selenium deficiency, reduced intracellular vitamin E levels, reduced glutathione system activity) and increased pro-oxidant activity associated with advanced age, diabetes, chronic inflammation, retained uremic solutes, and dialysis membranes and solutions.33 Four oxidative stress pathways can be hypothesized in CKD: carbonyl stress, nitrosative stress, chlorinated stress, and classical oxidative stress (Fig. 82-7).
Inflammation
Most dialysis patients are in a state of chronic inflammation; inflammatory biomarkers, such as CRP, interleukin (IL)-6, pentraxin 3 (PTX3), fibrinogen, and white blood cell count, are robust and independent predictors of mortality in CKD patients. Hypoalbuminemia, a biochemical factor strongly associated with systemic inflammation, is another strong outcome predictor in CKD. Whereas both dialysis-related factors and non–dialysis-related factors (infection, comorbidity, genetic factors, diet, renal function loss) may contribute to chronic inflammation, its primary causes are not always evident. As in the general population, it is unclear in CKD patients whether the acute-phase response reflects only established atherosclerotic disease or is involved in the initiation and progression of atherosclerosis. Some inflammatory biomarkers, such as IL-6, PTX3, and tumor necrosis factor, may have proatherogenic properties, such as promoting vascular calcification, oxidative stress, and endothelial dysfunction (Fig. 82-8). Evidence also suggests associations between inflammation and development of albuminuria. The link between septicemia and subsequent increased risk of death and cardiovascular events, including AMI, in observational studies further supports inflammation as a trigger for cardiovascular events.34
Endothelial Dysfunction
Endothelial dysfunction (as evaluated by impaired endothelium-dependent vasodilation) is a prominent feature of CKD. Reasons include inflammation, ADMA retention, oxidative stress, dyslipidemia, hyperglycemia, and hypertension. Plasma ADMA concentrations are associated with endothelial function in uremic resistance vasculature.35 Surrogate markers of endothelial dysfunction, such as ADMA, PTX3, and adhesion molecules, independently predict death.15 Detached circulating endothelial cells serve as potential markers of endothelial damage in renal and nonrenal patients and have prognostic value in HD patients.15 Normally, in response to ischemic insult and cytokine stimulation, endothelial progenitor cells are mobilized from the bone marrow to act as repair cells for the endothelial injury. Because CKD patients seem to have reduced numbers or functional impairment of endothelial progenitor cells as a result of inflammation or uremic toxins, they seem predisposed to endothelial dysfunction. An independent association exists between circulating fibroblast growth factor 23 (FGF23) and endothelial dysfunction.36
Anemia
Anemia is a major cause of LVH and LV dilation in CKD. Although partial correction of anemia with erythropoiesis-stimulating agents (ESAs) results in LVH regression, current information suggests no cardiovascular outcome benefit of normalized hemoglobin (see Chapter 83).
Secondary Hyperparathyroidism and Mineral Metabolism
Disturbances of calcium and phosphate metabolism starting as early as CKD stages 3 and 4 might accelerate calcifying atherosclerosis and arteriosclerosis. Recent evidence suggests that chronically elevated FGF23 levels contribute directly to high rates of LVH and mortality in CKD.37 In registry data, a strong independent mortality risk is predicted by hyperphosphatemia, an intermediate risk by elevated serum calcium levels, and a weak but significant risk by high or low serum intact parathyroid hormone levels. The overall mortality risk prediction attributable to mineral metabolism disorders is estimated to be about 17% in HD patients.38
Cardiovascular Calcification
Cardiovascular calcification may affect the arterial media, atherosclerotic plaques, myocardium, and heart valves. Medial calcification causes arterial stiffness and, consequently, increased pulse pressure. The pathophysiologic role of plaque calcification is less clear because soft plaques are assumed to rupture and to cause AMI; atherosclerotic calcification is a potent risk marker of cardiovascular events, but its utility as a risk marker for clinical management of CKD patients remains controversial. Valvular calcification mostly affects the aortic and mitral (annulus) valves in dialysis patients and contributes to progressive stenosis and associated morbidity; mitral annular calcification is associated with increased mortality.39 In dialysis patients, extensive vascular, especially coronary artery, calcification can occur at young ages. Calcium and phosphate metabolism disturbance is an important factor in cardiovascular calcification in ESRD. A serum phosphate level of 5.0 mg/dl or higher (≥1.62 mmol/l) is associated with increased risk of valvular heart surgery in HD patients.40 Calciphylaxis (calcific uremic arteriolopathy), characterized by severe calcifications of cutaneous arterioles and tissue necrosis, is discussed in Chapter 88.
Vascular calcification is not derived only from passive calcium and phosphate precipitation. Rather, it is a highly regulated active process involving differentiation of vascular smooth muscle cells toward osteoblasts induced by phosphate, calcium, and other factors, such as calcitriol and proinflammatory cytokines. One way by which chronic inflammation promotes vascular calcification may involve downregulation of fetuin-A, the most potent circulating inhibitor of extraosseous calcification and formation of calciprotein particles.41 In cross-sectional studies, dialysis patients with low serum fetuin-A levels showed significantly poorer survival than those with normal values.42 Apart from fetuin-A, other inhibitors probably counteract unwanted calcification. Leptin, matrix GLA protein, FGF23, pyrophosphates, bone morphogenic proteins (e.g., BMP-2 and BMP-7), and osteoprotegerin may be related to accelerated vascular calcification in ESRD. Recent studies indicate that deficiency of vitamin K and/or treatment with vitamin K antagonists (warfarin) may accelerate the vascular calcification process in the uremic milieu.43
Advanced Glycation End Products
Advanced glycation end products accumulate in CKD patients as a result of nonenzymatic glycation, oxidative stress, and diminished clearance of AGE precursors. Stable AGE residues of long-lived proteins are biomarkers of cumulative metabolic, inflammatory, and oxidative stress; carbonyl stress is speculated to contribute to tissue aging and long-term CKD complications. Whether AGE inhibition may be a useful intervention to reduce cardiovascular events, progression, and mortality in CKD is unknown.
Dialysis Modality
Reports from dialysis registries are inconsistent with regard to whether HD or PD is associated with better outcomes.44 Valid mortality comparisons between modalities would require stratification of patients according to underlying ESRD cause, age, and level of baseline comorbidity.44 Such data are currently not available.
Clinical Manifestations and Natural History
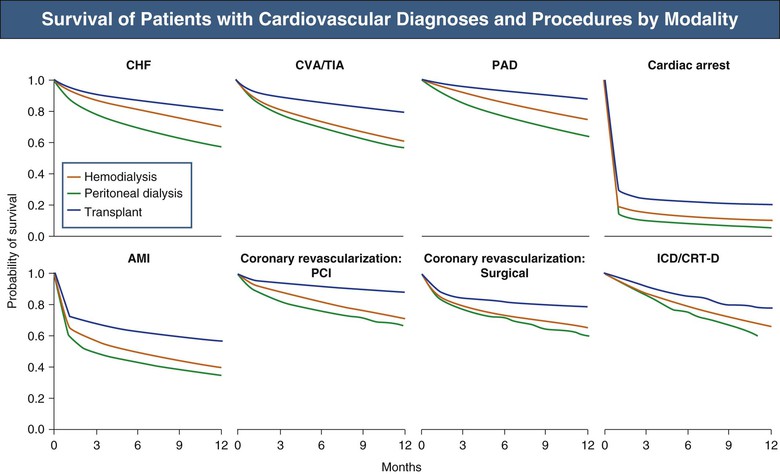
Figure 82-9 shows survival of patients with cardiovascular diagnoses and procedures, by renal replacement therapy modality.
Chest Pain, Coronary Heart Disease, and Acute Myocardial Infarction
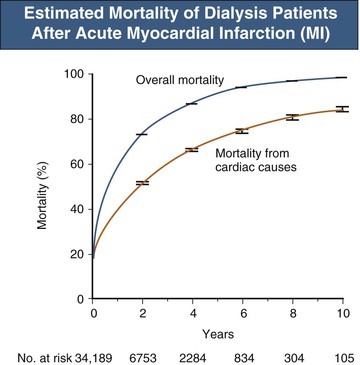
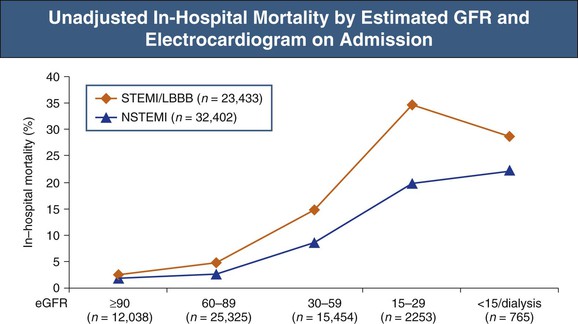
Acute myocardial infarction in dialysis patients is associated with poor long-term survival. The unadjusted 73% 2-year mortality rate has changed minimally in 25 years45 despite dramatic improvements in AMI outcomes in the general population (Fig. 82-10). This heightened mortality is not restricted to ESRD patients; in-hospital deaths increase with decreasing GFR (Fig. 82-11).46–48 Furthermore, the likelihood of prescribing evidence-based therapies, including aspirin and β-blockers, in CKD patients is historically diminished46–49 (but recently increased),1 although these therapies decrease mortality in such patients.
Prior speculations have attributed this poor outcome to under-recognition resulting from “atypical” presentations,49 underuse of appropriate diagnostic investigations, and undertreatment (therapeutic nihilism).49,50 The collaborative USRDS/National Registry of Myocardial Infarction study50 of dialysis patients hospitalized for AMI found that:
▪ 44% of dialysis patients, versus 68% of nondialysis patients, experienced chest pain.
▪ 19% of dialysis patients, versus 36% of nondialysis patients, had ST-segment elevation.
Similar findings occur across the CKD spectrum; the likelihood of increased mortality and lower prevalence of both ST-segment elevation myocardial infarction (STEMI) and chest pain are correlated with severity of non–dialysis-dependent CKD in patients with AMI.47,48,51 Patients with eGFR below 45 ml/min/1.73 m2 are three times as likely to present with AMI as with stable angina as the initial manifestation of CHD.52
Peripheral Arterial Disease
Risk of peripheral arterial disease (PAD) is highest for dialysis patients with diabetes or preexisting atherosclerosis. In HD patients, PAD is also associated with time on dialysis, hypoalbuminemia, low parathyroid hormone levels, and low predialysis diastolic blood pressure. Vascular medial calcification of large peripheral arteries may not indicate presence of occlusive disease, and peripheral gangrene is often caused by diabetic or other small-vessel disease or rarely by calcific uremic arteriolopathy (see Chapter 88). PAD is associated with increased mortality; outcomes after revascularization are worse than for the general population,53 in part reflecting advanced vasculopathy. About one fourth of CKD patients and half of HD patients have PAD,1,54 and the knowledge gaps regarding treatment of PAD in CKD patients are large.13
Cerebrovascular Disease and Atrial Fibrillation
Cognitive impairment is severe in more than a third of dialysis patients, and only 15% have normal cognition.55 Cognitive impairment prevalence increases approximately 10% per 10 ml/min/1.73 m2 of eGFR less than 60 ml/min/1.73 m2.56 Microalbuminuria and stage 3 CKD increased stroke risk 1.5-fold to 2-fold in a multivariate model.57,58 Incident dialysis patients have a sixfold age-adjusted relative risk of stroke compared with the general population; the unadjusted rate for stroke hospitalization for dialysis patients was 48 per 1000 patient-years, and 11% of these hospitalizations were attributed to hemorrhagic stroke.59 Dialysis initiation is associated with a fourfold to sevenfold increased stroke incidence compared with the rate 12 months before initiation.60 Stroke accounts for 3% of ESRD deaths in the USRDS registry1 (see Chapter 86 for more details).
Atrial fibrillation is the most common dysrhythmia in CKD patients; the prevalence among dialysis patients is 15% to 20%, and AF is associated with an increased stroke rate. Incident AF is an independent risk factor for development of ESRD in patients with CKD.61 The incidence of AF is high and apparently increasing in older patients initiating dialysis in the United States.12 Among warfarin-treated AF patients with stage 3 CKD, the hazard of stroke doubled versus lower CKD stages, and adjusted-dose warfarin was associated with a 76% reduction in the relative risk of ischemic stroke or systemic embolism.63 In AMI patients with AF, warfarin treatment was associated with a lower 1-year risk for the composite outcome of death, AMI, and ischemic stroke without a higher risk of bleeding, irrespective of CKD severity was observed.63a
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree