Eric S. Rovner, MD
Bladder Diverticula
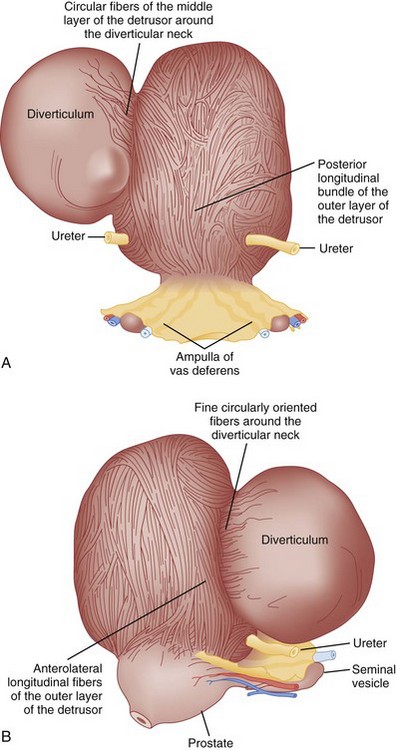
Bladder diverticula represent a herniation of the bladder urothelium through the muscularis propria of the bladder wall. This results in the typical finding of a variably sized, thin-walled, urine-filled structure adjacent to and connecting with the bladder lumen through a narrow neck, or ostium. Histologically, the diverticulum wall is composed of mucosa, subepithelial connective tissue or lamina propria, scattered thin muscle fibers, and an adventitial layer (Fig. 78–1) (Peterson et al, 1973; Gil-Vernet, 1998). A fibrous capsule or pseudocapsule outer shell is often present and may be a useful surgical plane for excision (see later discussion). The outside wall of the bladder diverticulum may contain some residual scattered strands or bundles of smooth muscle; however, these are disorganized and nonfunctional. Therefore bladder diverticula generally empty poorly during micturition, leaving a large postvoid residual urine volume that results in the characteristic findings on presentation and imaging.
Classification, Pathophysiology, and Etiology
Bladder diverticula may be classified as either congenital or acquired. Pathophysiology, presentation, and imaging may differentiate these two types. Congenital diverticula usually present during childhood, with a peak incidence in those less than 10 years old (Boechat and Lebowitz, 1978). These are usually solitary, occur most commonly in males (Stage and Tank, 1992; Sarihan and Abes, 1998; Evangelidis et al, 2005; Garat et al, 2007), and are located lateral and posterior to the ureteral orifice, often in association with vesicoureteral reflux (Evangelidis et al, 2005; Garat et al, 2007). The primary causation in those without coexisting lower urinary tract conditions appears to be a congenital weakness at the level of the ureterovesical junction and not bladder outlet obstruction (Johnston, 1960; Hutch, 1961; Hutch et al, 1961; Stephens, 1979). Blane and colleagues (1994) reported the incidence of bladder diverticula in children to be approximately 1.7% in a pediatric genitourinary database of more than 5000 cases. Congenital bladder diverticula are usually solitary but larger in comparison with those associated with obstruction or neurogenic bladder dysfunction (Gearhart, 2002). The most common presentation of congenital bladder diverticula is urinary tract infection (Bauer and Retik, 1974; Pieretti and Pieretti-Vanmarcke, 1999; Evangelidis et al, 2005; Garat et al, 2007). Congenital bladder diverticula may occur in the presence of normal voiding dynamics in the absence of bladder outlet obstruction (Cendron and Alain, 1972; Barrett et al, 1976), but secondary bladder outlet obstruction may occur when the diverticulum extends distally toward the bladder neck (Taylor et al, 1979; Epstein et al, 1982; Verghese and Belman, 1984; Oge et al, 2002). Typically, congenital bladder diverticula are found in smooth-walled bladders and are not associated with significant trabeculation on cystoscopic examination (Hutch, 1961). However, in patients with prune-belly syndrome or posterior urethral valves, bladder diverticula may be located at the dome and be associated with aberrant voiding dynamics and/or anatomy. These are to be distinguished from the urachal diverticula seen in some pediatric urologic conditions. Congenital bladder diverticula have been noted in association with a number of congenital syndromes, including Menkes syndrome (kinky hair or copper deficiency syndrome) (Harcke et al, 1977; Daly and Rabinovitch, 1981), Williams syndrome (Babbitt et al, 1979; Blane et al, 1994; Schulman et al, 1996), Ehlers-Danlos syndrome (Breivik et al, 1985; Levard et al, 1989; Schippers and Dittler, 1989; Rabin et al, 1991; Bade et al, 1994; Cuckow et al, 1994; Burrows et al, 1998), and fetal alcohol syndrome (Lewis and Woods, 1994). Whether there is a genetic predisposition to the formation of bladder diverticula in individuals without congenital syndromes is unclear, although congenital bladder diverticula have been reported in twins (Beall and Berger, 1978) and possibly as an autosomal-dominant trait in a family (Hofmann et al, 1984).
Acquired (also termed “secondary”) diverticula occur most commonly in the setting of bladder outlet obstruction or neurogenic vesicourethral dysfunction. Similar to the congenital type, these diverticula are also located most commonly at the ureterovesical hiatus (Van Arsdalen and Wein, 1992) but also occur elsewhere in the bladder. Acquired diverticula in males usually occur after age 60, which corresponds to the age of the development of prostatic enlargement (Fig. 78–2). Bladder outlet obstructions, including those due to benign and malignant disease of the prostate or urethral stricture, are commonly associated factors in adults, although obstruction is not considered to be present in all cases (Blacklock et al, 1983). Acquired diverticula are often multiple, typically found in association with significant bladder trabeculation (Wesselhoeft et al, 1963), and much more common in males than females (Senger et al, 1952; Pool and Hacker, 1966). Bladder diverticula in females are uncommon (Gillon et al, 1988) and quite rare in the absence of obstruction (Safir et al, 1998) (Fig. 78–3). Historically, the reported prevalence of moderate- to large-sized bladder diverticula in association with “prostatism” is approximately 1% to 6% (Burns, 1944). It is important to note that an acquired bladder diverticulum may also be found in children and young adults secondary to a number of conditions, including bladder neck dysfunction, posterior urethral valves, and neurogenic vesicourethral dysfunction. If the diverticulum encompasses the ureteral orifice in the setting of a neurogenic bladder and vesicoureteral reflux, it is termed a “Hutch” diverticulum (Hutch, 1952). These diverticula may also occur in the setting of dysfunctional voiding.
Bladder diverticula may also be iatrogenic (Hernandez et al, 1997; Suzuki et al, 2002; Chertin and Prat, 2008). Inadequate closure of the muscular layers of the bladder wall following a cystotomy for any indication may result in formation of a bladder diverticulum at a weak point of the suture line. Bladder diverticula may also occur following ureteral reimplantation surgery at the ureteroneocystostomy site (Ahmed and Tan, 1982; Sheu et al, 1998).
Diagnosis and Evaluation
Presentation
Acquired bladder diverticula do not typically produce specific symptoms. Because large bladder diverticula empty poorly or incompletely during voiding, symptoms and signs, if present, are usually attributed to urinary stasis within the diverticulum or, alternatively, to its mass effect in the lower abdomen and pelvis. Retrospectively, when queried, patient symptoms such as incomplete bladder emptying, lower abdominal fullness, and double voiding may be attributed to some large bladder diverticula. These symptoms, however, are nonspecific and can be due to prostatic enlargement, obstruction, or a number of other lower urinary tract conditions. Most bladder diverticula are found during the investigation of nonspecific lower urinary symptoms, hematuria, or infection—or, alternatively, noted incidentally during radiographic or endoscopic investigation of these conditions. As noted previously, congenital bladder diverticula most often present with urinary tract infection (Bauer and Retik, 1974; Pieretti and Pieretti-Vanmarcke, 1999; Evangelidis et al, 2005; Garat et al, 2007), but hematuria, abdominal pain, or an abdominal mass may be present as well. Inguinal hernias containing bladder diverticula have also been reported (Scardino and Upson, 1953; Bolton and Joyce, 1994; Buchholz et al, 1998; Schewe et al, 2000).
Urinary tract infection (UTI) in the male has been associated with bladder diverticula, because it is often an infection in a male that provides the impetus for further clinical investigation uncovering a diverticulum. A bladder diverticulum and subsequent urinary stasis may result in a predisposition for UTI and may also confer an increased difficulty in eradicating an existing infection (Shah, 1979; Taylor et al, 1979). Nevertheless, urine analysis and urine culture are important in the evaluation of bladder diverticula. Abnormalities of the urine sediment are common in patients with bladder diverticula. Pyuria and hematuria are often present. In fact, relapsing or persistent pyuria unresponsive to antibiotic therapy may be an indication for bladder diverticulectomy. Urine cytology should be considered in most patients with bladder diverticula, especially when nonoperative management is being considered.
Imaging
The diagnosis of bladder diverticula relies on radiographic and endoscopic findings. Bladder diverticula are part of the radiologic continuum that includes cellules and saccules. Cellules, saccules, and bladder diverticula are felt to represent increasingly larger, therefore more severe manifestations of the same pathologic process involving elevated intravesical voiding pressure (Talner et al, 2000). Cellules and saccules represent small outpouchings between hypertrophied bands of bladder muscle, with saccules generally being larger than cellules. However, there are no universally agreed upon minimum size requirements between cellules, saccules, and bladder diverticula, and the differentiation between these three entities is often subjective and arbitrary (Talner et al, 2000).
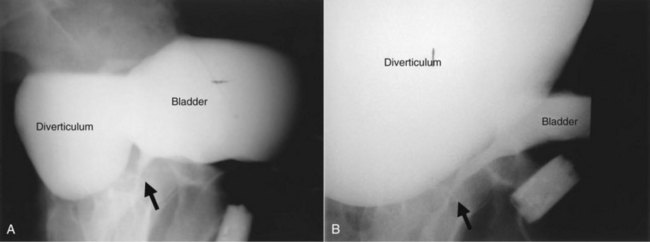
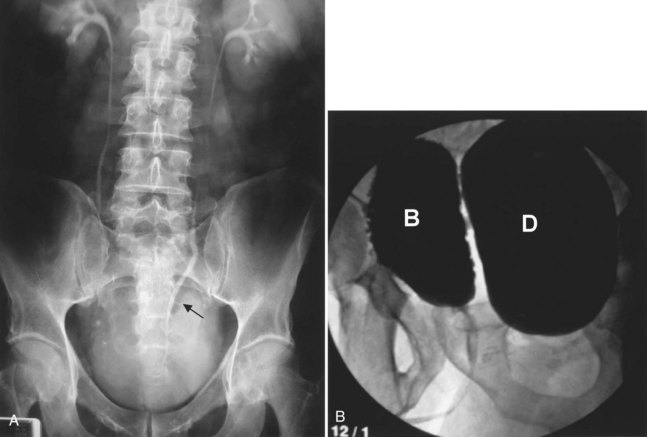
As noted previously, bladder diverticula are often found incidentally in the radiographic investigation of recurrent urinary tract infections or other nonspecific lower urinary tract symptoms or signs. Fluoroscopically monitored voiding cystourethrography is an excellent study to detect bladder diverticula; however, false negatives are possible (Hernanz-Schulman and Lebowitz, 1985). A voiding urethrocystogram (VCUG) with anterior-posterior, oblique, and lateral images provides information regarding anatomy, location, and size and also associated vesicoureteric reflux and, importantly, emptying of the bladder diverticulum with voiding. Anomalous voiding into the diverticulum during a detrusor contraction may result in paradoxical enlargement of the bladder diverticulum during micturition (Wesselhoeft et al, 1963) (see Fig. 78–2). Presumably, this occurs as the contrast flows from an area of relatively high pressure in the bladder, during the detrusor contraction, to the diverticulum, which represents an area of low pressure. In some instances, the bladder may empty partly into the diverticulum and partly through the urethra. The amount of postvoid residual urine in the bladder diverticulum and bladder should be noted. Incomplete bladder emptying suggests that a urodynamic abnormality may be present, requiring further study.
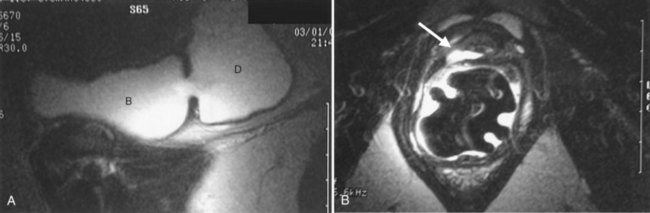
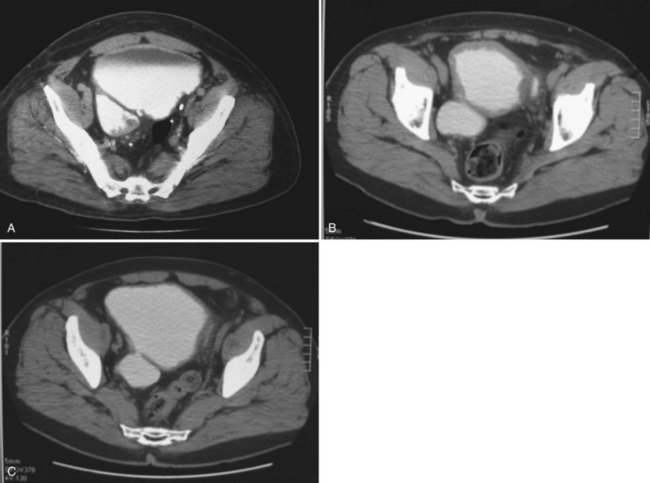
If the neck of the diverticulum is obstructed from a tumor or otherwise not patent, cross-sectional imaging may be required for diagnosis (Dondalski et al, 1993). Cross-sectional imaging, including computed tomography (CT) and magnetic resonance imaging (MRI) may also be useful for the evaluation of masses within the diverticulum (see under Associated Conditions). Review of the radiographic films should accurately characterize the number, anatomy, and location of the diverticula. Filling defects within the diverticula or other bladder abnormalities should prompt further investigation (Fig. 78–4).
Imaging of the upper urinary tract may include intravenous pyelography (IVP), ultrasonography, CT, or MRI. In the absence of hematuria or a known or suspected urinary tract malignancy, the goal of upper tract imaging is to evaluate for asymptomatic or silent hydroureteronephrosis related to the diverticulum (Lebowitz et al, 1979; Sharma et al, 1997). Hydronephrosis may be related to obstruction of the ureter (Kwan and Lowe, 1992; Livne and Gonzales, 1985), an underlying urodynamic abnormality that resulted in the formation of the diverticulum, vesicoureteral reflux in association with the diverticulum, inflammation (Bellinger et al, 1985), or may be completely unrelated to the bladder diverticulum. A multichannel urodynamic study in combination with a voiding cystourethrogram (VCUG) or, alternatively, a videourodynamic study is useful in this setting to differentiate upper from lower urinary tract causes of the hydronephrosis.
A bladder diverticulum may cause deviation with or without compression of the ipsilateral ureter. On intravenous urography, medial deviation of the pelvic ureter is most commonly seen; however, lateral deviation may also occur (Talner et al, 2000) (Fig. 78–5). Furthermore, a bladder diverticulum that encompasses the ureteral orifice may create a functionally shortened intramural ureteral segment and result in vesicoureteric reflux (see later discussion). In these patients, excision of the bladder diverticulum with ureteroneocystotomy may be necessary.
Urodynamics
Bladder outlet obstruction, impaired compliance, and/or neurogenic voiding dysfunction may result in the formation of bladder diverticula. Therefore a urodynamic study can be very helpful in the investigation of these patients. Failure to identify and treat an existing underlying urodynamic abnormality prior to or, concomitantly, with definitive surgical therapy for bladder diverticula may result in a high risk of recurrent diverticula or other problems following surgery. Successful treatment of the urodynamic abnormality may improve bladder emptying and may potentially result in resolution of the symptoms and/or complications. Importantly, bladder contractility may appear to be diminished on urodynamics due to the “pressure sink” effect of the bladder diverticulum. This artifact occurs as the detrusor contracts and the intravesical contents are decompressed through the path of least resistance into the bladder diverticulum as opposed to the urethra (Wilson and Klufio, 1985). Nevertheless, when carefully measured, bladder contractility is not significantly different from those individuals with benign prostatic hyperplasia (BPH) without bladder diverticula (Adot Zurbano et al, 2005).
Endoscopic Examination
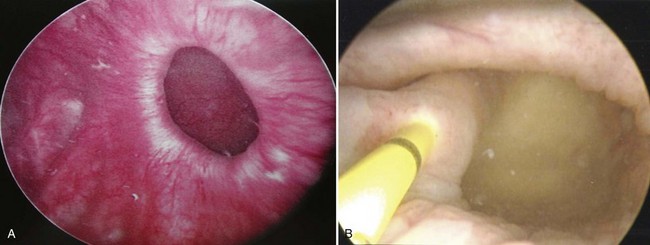
Endoscopically, the entire interior of each bladder diverticulum should be thoroughly inspected for stones or abnormal-appearing epithelium. Flexible cystoscopy is often required to examine the entire interior of some diverticula. The location of the diverticula relative to the ureters and bladder outlet is noted, and the size of the diverticulum is carefully recorded (Fig. 78–6). Ideally, urine cytology should be obtained from the diverticulum during endoscopic examination. Any abnormal-appearing epithelium or lesions within the diverticulum are carefully biopsied. Extreme care must be taken during the biopsy to prevent perforation, because the wall of the diverticulum is very thin due to the lack of a muscularis propria layer. Patients who elect nonoperative management of bladder diverticula are usually followed closely with periodic endoscopic examinations and cytology. The natural history of untreated bladder diverticula is unknown, but the possibility of malignant transformation over time due to urinary stasis within the diverticulum should be considered.
Associated Conditions
The finding of a neoplasm within a bladder diverticulum has particularly important diagnostic and therapeutic considerations. The overall prevalence of malignant tumors within a bladder diverticulum has been reported as ranging from 0.8% to 10% (Montague and Boltuch, 1976; Micic and Ilic, 1983; Melekos et al, 1987; Baniel and Vishna, 1997; Golijanin et al, 2003). However, it is likely that the prevalence of bladder diverticula overall is under-reported and, as such, the prevalence of tumors within diverticula is probably considerably smaller than that reported above. Nevertheless, the most common histologic type of malignancy seen within bladder diverticula is transitional cell carcinoma in approximately 70% to 80% of cases, followed by squamous cell carcinoma in 20% to 25% of cases (Montague and Boltuch, 1976; Redman et al, 1976; Micic and Ilic, 1983; Van Arsdalen and Wein, 1992; Yu et al, 1993; Baniel and Vishna, 1997; Golijanin et al, 2003). Tumors in bladder diverticula occur almost exclusively in adults with a peak occurrence between ages 65 and 75 (Ostroff et al, 1973). The prognosis for patients with these tumors has been historically reported as poor (Faysal and Freiha, 1981; Micic and Ilic, 1983; Das and Amar, 1986; Melekos et al, 1987), although some reports may suggest otherwise in selected patients (Montague and Boltuch, 1976; Baniel and Vishna, 1997). The reason for the poor prognosis has been attributed to delayed diagnosis and advanced stage at presentation (Ostroff et al, 1973; Fellows, 1978; Faysal and Freiha, 1981; Micic and Ilic, 1983) in some patients. The lack of a well-defined muscularis layer of bladder diverticula is likely to be a very important factor. Because bladder diverticula lack a muscular layer beyond the urothelium, there is a theoretically greater risk for early transmural involvement with local extension to the perivesical fat through the comparatively thinner diverticular wall as compared with the normal bladder wall. In addition, the lack of a defined muscular wall risks early dissemination of tumor cells into an extravesical location during transurethral resection of these lesions and makes precise pathologic staging difficult. Accurate identification of tumors within bladder diverticula has been demonstrated using a variety of radiographic techniques, including CT, MRI, and ultrasonography (Dragsted and Nilsson, 1985; Saez et al, 1985; Williams and Gooding; 1985; Lowe et al, 1989; Dondalski et al, 1993; Durfee et al, 1997; Mallampati and Siegelman, 2004) (see Fig. 78–4). Whether these modalities can reliably predict extravesical invasion and therefore affect staging and prognosis is not clear. It has been suggested that clinical stage at presentation is the most important prognostic factor for patients with tumors in bladder diverticula, with 5-year actuarial survival ranging from 83 ± 9% in patients with superficial disease to 45 ± 14% in those presenting with extradiverticular disease (Golijanin et al, 2003). One small series suggests that aggressive individualized multimodal therapy in these patients, including surgery, chemotherapy, and radiation therapy may improve prognosis (Garzotto et al, 1996). Because pathologic staging following transurethral resection is difficult and often inaccurate, some authors have suggested a very aggressive approach to these tumors. This surgical staging approach involves an open exploration and partial or radical cystectomy without a prior transurethral resection (Redman et al, 1976). Others have advocated a selective individualized approach, taking into account the clinical stage and pathologic grade of the tumor (Golijanin et al, 2003). Low-grade, low-stage tumors may be successfully treated with diverticulectomy alone (Baniel and Vishna, 1997; Sulaiman et al, 1998); however, it is important to recognize that the ability to reliably predict stage and grade preoperatively is limited, and therefore this approach should be undertaken only with caution in select cases, with adequate counseling and follow-up. In any case, close surveillance of these patients is warranted whether or not aggressive therapy is undertaken.
The location of many bladder diverticula at the level of the ureterovesical junction may explain the high incidence of associated ipsilateral ureteral abnormalities. For a ureter found within a diverticulum, the lack of a muscular backing to the diverticulum results in a functionally shortened intramural tunnel (see Fig. 78–6B). A very high prevalence of ipsilateral vesicoureteral reflux has been noted in association with congenital bladder diverticula (Amar, 1972). One study noted reflux in 83 of 89 diverticulum associated ureteric units (Barrett et al, 1976). Other considerations in the evaluation and management of bladder diverticula include the potential development of stones within the diverticulum, ureteral obstruction (Lebowitz et al, 1979; Bellinger et al, 1985; Kwan and Lowe, 1992; Khan et al, 1994; Sharma et al, 1997), and even the potential for the rare but life-threatening complication of perforation and/or rupture of the bladder diverticulum (Mitchell and Hamilton, 1971; Keeler and Sant, 1990; Itoh and Kounami, 1994; Jorion and Michel, 1999).
Key Points: Bladder Diverticula—Diagnosis
Management
In general, if the etiology of the bladder diverticulum is felt to be consistent with bladder outlet obstruction, definitive treatment of the bladder outlet is indicated prior to formal diverticulectomy. This approach allows reassessment of the diverticulum following relief of outlet obstruction and, if bladder emptying is satisfactory, then the diverticulum may not require excision. However, simultaneous open bladder diverticulectomy/open prostatectomy, as well as simultaneous laparoscopic bladder diverticulectomy/transurethral prostatectomy (TURP) can be performed (Porpiglia et al, 2004). If treatment of the obstruction is pursued initially and results in satisfactory emptying of the diverticulum postoperatively, and complicating factors such as reflux, infection, malignancy, or stones are absent, then ongoing surveillance of the diverticulum may be all that is required. Emptying of the diverticulum following relief of outlet obstruction may be assessed on ultrasonography, VCUG, or by videourodynamics.
Management options in the treatment of bladder diverticula include observation, endoscopic management, and surgical excision; however, there are many factors to consider during the evaluation of these lesions prior to deciding on appropriate therapy (Fig. 78–7).
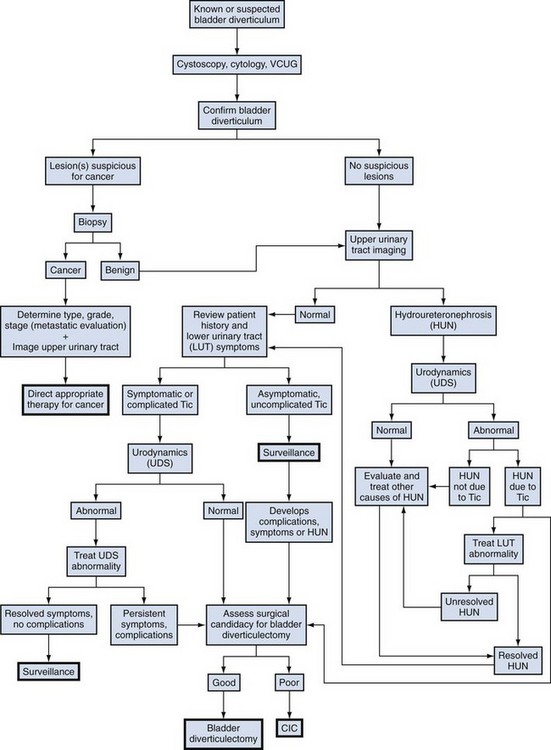
(Adapted from Rovner ES, Wein AJ. Bladder diverticula in adults. In: Resnick M, Elder JA, Spirnak JP, editors. Decision making in urology. 3rd ed. Hamilton [Ontario]: B.C. Decker; 2004. p. 260–3.)
Endoscopic Management
Endoscopic management of bladder diverticulum may be considered in patients who are aged or somewhat debilitated, those who are not good candidates for an open operative approach, or for those undergoing transurethral resection of prostate in whom there exists an associated poorly draining diverticulum (Orandi, 1977; Vitale and Woodside, 1979). Transurethral resection of the diverticular neck has been reported to be successful in select cases (Vitale and Woodside, 1979). This technique is performed with a standard resectoscope. The neck of the diverticulum is incised using the resectoscope loop or Collins knife. Incisions are carried down to the muscular fibers of the bladder at the level of the ostium of the diverticulum. Circumferential resection of the entire neck may be performed. When successful, this procedure enlarges the neck of the diverticulum, disrupting the narrow sphincteric-like properties of its connection to the bladder lumen, thereby permitting improved emptying of the diverticulum during micturition. Although generally safe and well tolerated, this technique has resulted in urinary retention due to a reversal of flow during micturition and “venting” of the bladder contents into the diverticulum postoperatively (Schulze and Hald, 1983). Transurethral resection of the diverticular neck may be combined with fulguration of the entire urothelial lining of the diverticulum (Clayman et al, 1984; Adachi et al, 1991a, 1991b; Yamaguchi et al, 1992). Fulguration of the lining of the diverticulum should result in obliteration of the diverticulum or a considerable reduction in its size.
Open Surgical Management
Open excision is usually performed through a transvesical approach, although extravesical and combined approaches have been described. In cases of marked prostatic enlargement and obstruction, open suprapubic prostatectomy and transvesical bladder diverticulectomy may be performed concomitantly. Adequate preoperative imaging and endoscopic evaluation should provide information regarding the size, number, and location of all bladder diverticula. Furthermore, the relationship of the adjacent anatomic structures, including the major pelvic vessels, ureters, and bowel, should be noted. Surgically, the bladder is most commonly approached extraperitoneally through a low midline or transverse incision. The retropubic space is entered and developed. Regardless of the technique used, careful dissection is required during excision of the diverticulum to avoid ureteral injury, because many bladder diverticula are located adjacent to the ureter or may be adherent to it. Ureteral stents are often placed preoperatively or intraoperatively to facilitate dissection and avoid ureteric injury. Often, several bladder diverticula are noted preoperatively. Simultaneous resection of all existing bladder diverticula should be performed to optimize postoperative bladder emptying (Gil-Vernet, 1998).
Transvesical Bladder Diverticulectomy
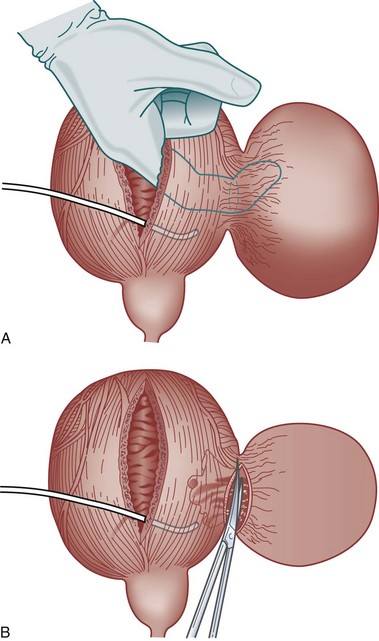
The transvesical approach to bladder diverticulectomy was first reported by Hugh Hampton Young in 1906 (Gil-Vernet, 1998). The bladder is opened along the anterior wall and suitable retraction is placed in order to visualize the neck of the diverticulum. For the excision of small diverticula, saccules or cellules, a locking instrument such as an Allis-type clamp is passed through the neck of the diverticulum, and the base or bottom of the diverticulum is grasped, pulled, and everted back into the bladder. In the absence of extravesical adhesions or inflammation, a small diverticulum can be completely everted into the bladder and exposed in this manner. The urothelium of the diverticulum at the level of the neck is incised circumferentially, and the diverticulum is removed. The defect in the bladder wall is closed with absorbable suture in two layers. Care must be taken when performing this technique to avoid injuring structures adherent or adjacent to external surface of the diverticulum, because they may be blindly pulled into the bladder with the diverticulum wall and inadvertently injured during resection.
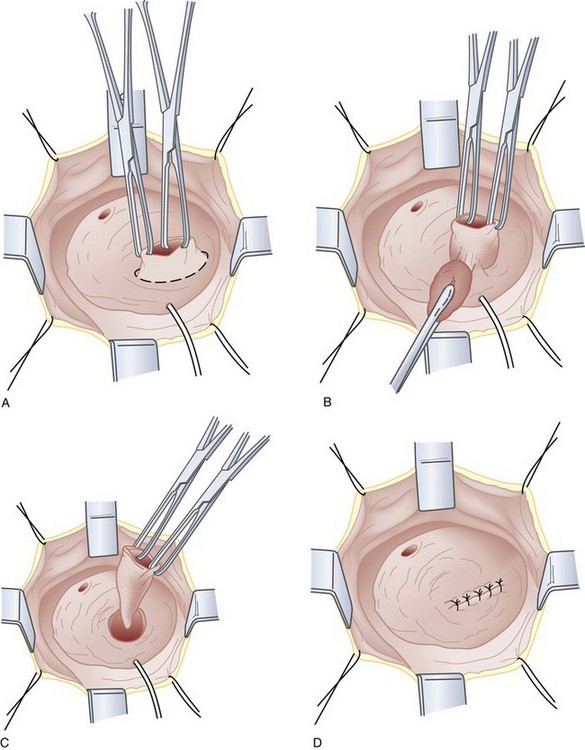
If eversion of the diverticulum is not possible due to adhesions or inflammation, or the diverticulum is too large to permit complete exposure during eversion, submucosal excision of the diverticulum may be performed. An anterior cystotomy is performed, the bladder is opened and suitable retraction is placed to visualize the neck of the diverticulum within the bladder. The neck of diverticulum is then identified and mobilized initially, similar to the technique used for the first part of the dissection of the ureter during ureteroneocystostomy. The diverticular neck is circumscribed sharply with scissors or electrocautery, and the plane between the wall of the diverticulum and the surrounding fibrous capsule is defined (Fig. 78–8A to D). Traction is placed on the edges of the neck of the diverticulum with Allis clamps circumferentially, and the neck and then the exterior walls of the diverticulum are carefully mobilized from the surrounding tissues and delivered into the bladder lumen. Sharp and blunt dissection on the exterior wall of the diverticulum is performed in a well-defined periadventitial plane between the diverticulum wall and the fibrous pseudocapsule. Packing of the diverticulum with gauze may facilitate dissection and provide some countertraction (Fig. 78–9A to D). The diverticulum is completely freed from its fibrous pseudocapsule and removed. The bladder wall defect is then repaired in two layers with absorbable sutures. Drainage of the potential space left by the pseudocapsule is usually not necessary (Firstater and Farkas, 1977).
Combined Intravesical/Extravesical Approach
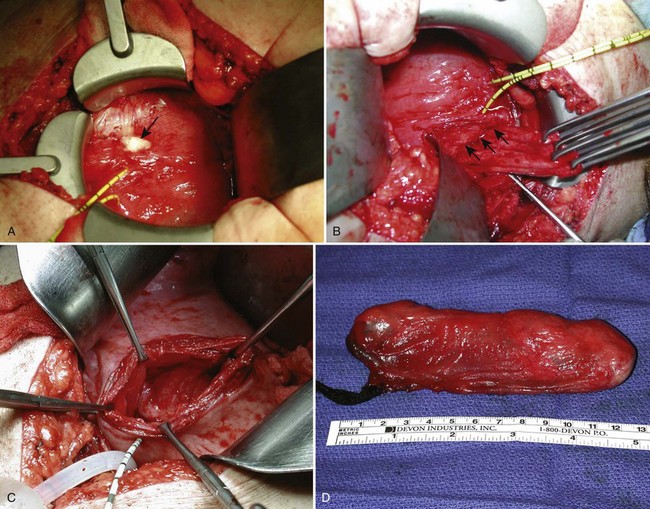
For individuals with large diverticula and/or considerable peridiverticular inflammation, a purely transvesical approach may not be feasible. In addition, involvement of the ureter within the diverticulum, or severe peridiverticular inflammation encompassing the ureter may have altered the usual course of the ureter and may incur a prohibitive risk of injuring the ureter with a transvesical approach. Therefore a combined intravesical and extravesical approach may be warranted. Ureteral catheters are usually placed to prevent ureteral injury. The bladder is opened as if for a transvesical diverticulectomy. The diverticular neck is incised circumferentially. The surgeon’s finger is then inserted into the diverticulum to identify the location of the rest of the diverticulum (Fig. 78–10). The diverticulum may be packed with gauze as well. If the neck of the diverticulum is sufficiently mobile, the anterior aspect of the neck is brought up into the operative field outside the bladder using the surgeon’s finger. The overlying tissue is dissected free, and the anterior portion of the neck of the diverticulum is exposed and incised extravesically. The neck is circumferentially mobilized, and then transected. The diverticular wall is then mobilized and dissected free of its attachments as described previously. In some cases, anterior reflection of the surgeon’s finger allows opening of the diverticulum itself, and dissection can be carried out in the plane between the pseudocapsule and the urothelium in a completely extravesical fashion. In some cases, it may be necessary to divide the ipsilateral superior vesical pedicle to facilitate exposure and delivery of the diverticulum. If additional difficulty is encountered in exposing the diverticulum, the original cystotomy incision can be extended or enlarged in a “T” fashion over to the neck of the diverticulum. This procedure generally removes the entire diverticulum, including the mucosa and fibrous pseudocapsule. In cases where considerable inflammation is encountered and the diverticulum is closely adhering to adjacent vital structures, it may be necessary to leave portions of the fibrous pseudocapsule in situ. The bladder is closed as described previously.
Laparoscopic and Robotic Diverticulectomy
Minimally invasive techniques, such as laparoscopy and robotics (Myer and Wagner, 2007), have been applied to surgical diverticulectomy using a similar surgical approach (Das, 1992; Parra et al, 1992; Jarrett et al, 1995; Faramarzi-Roques et al, 2004; Abdel-Hakim et al, 2007; Macejko et al, 2008). Intra- and extraperitoneal approaches have been reported. Approaching the diverticulum extravesically using such a minimally invasive approach can be challenging; however, simultaneous intraoperative cystoscopic transillumination may aid in locating the diverticulum within the retroperitoneal tissues (Macejko et al, 2008). Small, non-randomized case series suggest that outcomes are similar to established open techniques (Porpiglia et al, 2004; Myer and Wagner, 2007).
Complications
Key Points: Bladder Diverticula—Management
Female Urethral Diverticula (UD)
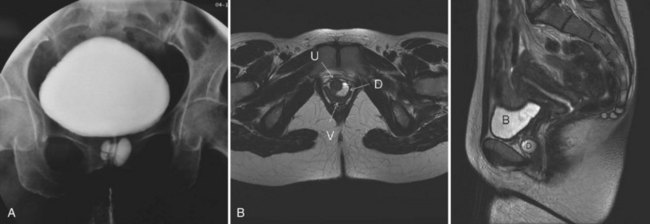
Although described as early as the 19th century (Hey, 1805), the modern era of female urethral diverticula (UD) began in 1956 with the advent of positive-pressure urethrography by Davis and Cian (1952). Over the next several years, there was a dramatic increase in number of cases of UD reported in the literature. A published series of 121 cases by Davis and Telinde (1958) approximately doubled the number of cases reported during the previous 60 years. Development of adjuvant imaging techniques, such as ultrasonography and surface coil/endoluminal MRI, in the past 2 to 3 decades have contributed greatly to further understanding of UD (Fig. 78–11).
Anatomy of the Female Urethra
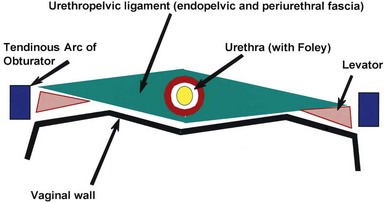
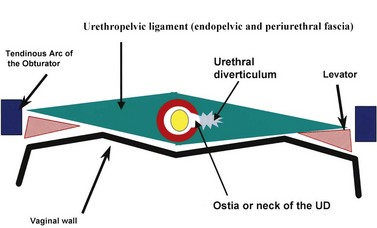
The normal female urethra is a musculofascial tube approximately 3 to 4 cm in length, extending from the bladder neck to the external urethral meatus, suspended to the pelvic sidewall and pelvic fascia (tendinous arc of the obturator muscle) by a sheet of connective tissue termed the urethropelvic ligament. The urethropelvic ligament is composed of two layers of fused pelvic fascia that extend toward the pelvic sidewall bilaterally (Fig. 78–12). This structure can be considered to have an abdominal side (the endopelvic fascia) and a vaginal side (periurethral fascia). Within and between these two leaves of fascia lies the urethra and the location of most urethral diverticula.
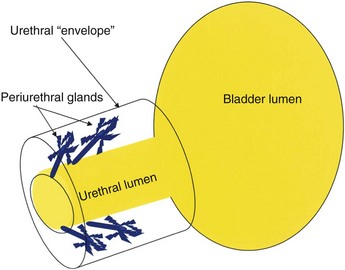
The urethral lumen is lined by an epithelial layer that is a transitional cell type proximally and nonkeratinized stratified squamous cell type distally. The urethra may be conceptualized as a rich, vascular, spongy cylinder surrounded by an envelope consisting of smooth and skeletal muscle and fibroelastic tissue (Young et al, 1996). Within the thick, vascular lamina propria/submucosal layer are the periurethral glands (Fig. 78–13). These tubuloalveolar glands exist over the entire length of the urethra posterolaterally; however, they are most prominent over the distal two thirds, with the majority of the glands draining into the distal one third of the urethra. Skene glands are the largest and most distal of these glands. These glands drain outside the urethral lumen, lateral to the urethral meatus. It is from pathologic processes involving the periurethral glands that most acquired female urethral diverticula are thought to originate.
The urethra has several muscular layers: an internal longitudinal smooth muscle layer, an outer circular smooth muscle layer, and a skeletal muscle layer. The skeletal muscle component spans much of the length of the urethra but is more prominent in the middle third. It has a U-shaped configuration, being deficient dorsally. Ventral to the urethra, but separated from it by the periurethral fascia, lies the anterior vaginal wall (see Fig. 78–12). The location and competence of the urethral sphincters have important implications when considering surgical repair of urethral diverticula due to the anatomic overlap of these two entities.
Arterial inflow to the urethra derives from two sources. The proximal urethra has a blood supply similar to the adjacent bladder, whereas the distal urethra derives its blood supply from the terminal branches of the inferior vesical artery through the vaginal artery that runs along the superior lateral aspect of the vagina (Hinman, 1993). Lymphatic drainage of the female urethra is to the external and internal iliac nodes from the proximal urethra, and to the superficial and deep inguinal lymph nodes from the distal urethra. Innervation to the female urethra is from the pudendal nerve (S2-S4), and afferents from the urethra travel through the pelvic splanchnic nerves.
Urethral Diverticula
Pathophysiology and Etiology
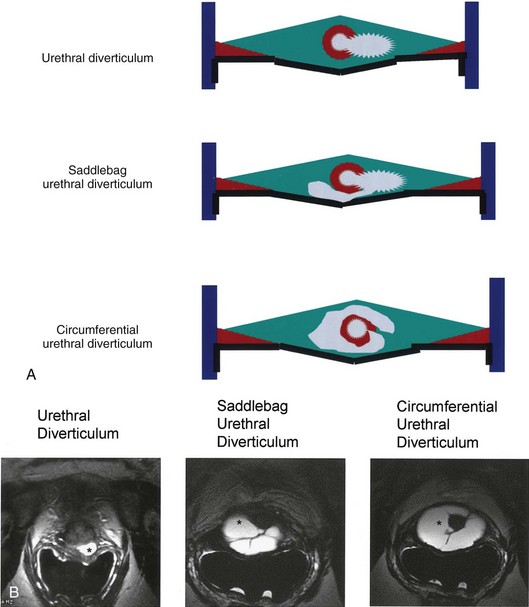
As conceptualized by Young and colleagues (1996), urethral diverticula represent a cavity dissecting within the confines of the fascia of the urethropelvic ligament. This defect is often an isolated cystlike appendage with a single discreet connection to the urethral lumen termed the neck, or ostium (Fig. 78–14). However, complicated anatomic patterns may exist, and in certain cases the UD may extend partially (“saddlebag” UD) around the urethra, anterior to the urethra (Vakili et al, 2003), or circumferentially around the entire urethra (Rovner and Wein, 2003) (Fig. 78–15).
The exact origin of UD is still unproven. A major debate in the earlier part of the 20th century focused on whether UD were congenital or acquired lesions (Johnson, 1938; Gilbert and Rivera-Cintron, 1954; Pinkerton, 1963). Although this condition exists in children, the diagnosis may represent a different clinical entity from adult female UD. Scattered reports of congenital UD in female infants have been described (Glassman et al, 1975). Marshall (1981) reported five cases of UD in young females, of whom three underwent spontaneous regression. Congenital anterior urethral diverticulum is a well-described entity in boys (Kirks and Grossman, 1981; Lau and Ong, 1981; Kaneti et al, 1984), but this is considered to be an entirely different clinical entity from UD in the female. Congenital Skene gland cysts have been reported (Kimbrough and Vaughan, 1977; Lee and Kim, 1992) but are considered extremely rare. Diverticula in the pediatric population have been attributed to a number of congenital anomalies, including an ectopic ureter draining into a Gartner duct cyst and a form fruste of urethral duplication (Silk and Lebowitz, 1969; Vanhoutte, 1970; Boyd and Raz, 1993). The vast majority of UD, however, are likely to be acquired and are diagnosed in adult females. In two large series of UD, there were no patients reported who were younger than 10 years of age (Davis and Robinson, 1970; Davis and TeLinde, 1958), arguing against a congenital etiology for these lesions. Although it is possible that there exists a congenital defect in patients that results in or represents a precursor to UD, which then only becomes symptomatic later in life, it is still unproven.
There are multiple theories regarding the formation of acquired UD. For many years, acquired UD were felt to be most likely due to trauma from vaginal childbirth (McNally, 1935). It was postulated that mechanical trauma during vaginal delivery resulted in herniation of the urethral mucosa through the muscular layers of the urethra with the subsequent development of a UD. However, up to 20% to 30% of patients in some UD series are nulliparous (Lee, 1984; Ganabathi et al, 1994), which may significantly discount parity as a risk factor. Trauma with forceps delivery, however, has been reported to cause UD (Klyszejko et al, 1985), and the radiographic appearance of periurethral injectable agents, including collagen (Clemens and Bushman, 2001; Bridges et al, 2005) and other injectable agents (Castillo-Vico et al, 2007), can simulate a urethral diverticulum.
The periurethral glands are felt to be the probable site of origin of acquired UD (Young et al, 1996). Huffman’s anatomic work with wax models of the female urethra was critical to the early theories regarding the pathophysiology of UD and the involvement of the periurethral glands (Huffman, 1948). By reviewing 10-µm transverse sections, he refuted earlier anatomic descriptions of the glandular anatomy of the female. He characterized the periurethral glands as located primarily dorsolateral to the urethra, arborizing proximally along the urethra, and yet draining into ducts located in the distal one third of the urethra (see Fig. 78–13). Furthermore, he noted that periductal and interductal inflammation was found commonly. In support of these observations and an infectious (acquired) etiology of UD, in over 90% of UD cases, the ostium is located posterolaterally in the mid- or distal urethra, which corresponds to the anatomic location of the periurethral glands (Lang and Davis, 1959; MacKinnon et al, 1959).
Although there are probably other unknown factors that may facilitate the initiation, formation or propagation of UD, infection of the periurethral glands seems to the most generally accepted common etiologic factor in most cases. Peters and Vaughan (1976)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree