Chapter 17 Benign Strictures
Esophageal Strictures
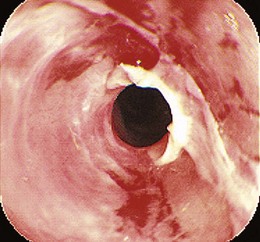
The most common presenting symptom of an esophageal stricture is solid food dysphagia. Although there continues to be some debate about whether a barium esophagogram or upper endoscopy is the best initial test in patients with dysphagia,1 many gastroenterologists favor endoscopy because the diagnoses that benefit from a barium x-ray, such as achalasia and Zenker’s diverticulum, are uncommon, and endoscopy offers both diagnosis and treatment for most patients. Dysphagia can be a symptom of gastrointestinal reflux disease (GERD) without the presence of a stricture.2 Dysphagia typically occurs when the esophagus narrows to a diameter of 13 mm or less (≤39-Fr). Mild degrees of stenosis can be missed endoscopically, and patients with persistent dysphagia after a normal endoscopy and a therapeutic trial of a proton pump inhibitor (PPI) should have a barium esophagogram subsequently performed. The most common etiology of benign esophageal stricture is GERD (Fig. 17.1).
Because of the widespread use of PPIs, peptic strictures are becoming less common3 and are recurring less frequently.4 They are usually at their worst at the initial presentation.5 Another etiology of esophageal strictures is corrosive (or caustic) strictures, resulting from either alkali or strong acid ingestion. Compared with peptic strictures, corrosive strictures require more dilation sessions, and the chance of recurrence is higher.6 Radiation-induced, infection-induced,7 pill-induced,8 sclerotherapy-induced,9 and eosinophilic esophagitis–induced esophageal strictures are less common (Table 17.1).10 Extrinsic compression of the esophagus can also cause symptomatic esophageal stenosis. The most common causes of extrinsic esophageal compression are mediastinal tumors, such as breast and lung cancer and lymphoma, although compression by lymph nodes in tuberculosis and histoplasmosis and by vascular structures such as an aberrant right subclavian artery (arteria lusoria)11,12 also occurs.
Table 17.1 Benign Diseases That Cause Dysphagia
| Mucosal disease | Gastrointestinal reflux disease (peptic stricture) |
| Caustic injury (corrosive ingestion, sclerotherapy) | |
| Radiation injury | |
| Pill-induced esophagitis | |
| Rings and webs | |
| Infectious esophagitis | |
| Eosinophilic esophagitis | |
| Anastomotic strictures | |
| Motility disorders | Achalasia |
| Scleroderma or CREST syndrome | |
| Hypothyroidism | |
| Other motility disorders | |
| Mediastinal compression | Mediastinal infections (tuberculosis, histoplasmosis) |
| Arteria lusoria |
CREST, calcinosis, Raynaud’s phenomenon, esophageal dysmotility, scleroderma, and telangiectases.
Dilation of Esophageal Strictures
Most reported series on the treatment of benign esophageal strictures are composed predominately or exclusively of patients with peptic strictures. Consequently, published guidelines on the management of strictures are based primarily on the results of studies of patients with peptic lesions.1,13
There are three primary types of dilator (Table 17.2). The first, and historically most widely used, type comprises the mercury-filled or tungsten-filled rubber bougies, either blunt-tipped (Hurst) or with a tapered tip (Maloney). Except for home dilation performed by patients, weighted bougies have been replaced by wire-guided, tapered, polyvinyl bougies or by balloons in most endoscopy units. The major advantage to the wire-guided bougie is the security of knowing that the tip is directed through the stricture rather than into a side wall. This security is heightened by the use of fluoroscopy for wire-guided bougie dilation, although fluoroscopic guidance is unnecessary unless the stricture is extremely narrow or tortuous. There are several manufacturers of wire-guided bougies. The Celestin dilator has a series of short steps, which increase the diameter in a stepwise manner, rather than a smooth taper. In the United States, the Savary (Wilson-Cook) and American Endoscopy (Bard) bougies are the most popular. They differ in the length of the taper and the method for making them radiopaque. Available diameters range from 5 to 20 mm (15-Fr to 60-Fr).
Table 17.2 Esophageal Dilators
| Mercury- or tungsten-filled bougies | Maloney (tapered tip) |
| Hurst (blunt tip) | |
| Wire-guided polyvinyl bougies | Savary |
| American Endoscopy | |
| Celestin (stepwise diameter increase) | |
| Balloon dilators | Through the scope (TTS) |
| Controlled radial expansion (CRE) through the scope | |
| Over-a-wire fluoroscopic control |
There is insufficient evidence to support claims that fluoroscopic guidance improves the safety of esophageal dilation.14 In a study of 145 patients treated with Maloney dilators, fluoroscopy was found to alter the dilation technique in 24%.15 Fluoroscopy was found to be particularly useful for ensuring proper dilator passage in patients with large hiatal hernias. However, the wire-guided bougie alleviates the need for fluoroscopic control. Only in very tight, long, or tortuous strictures where the wire does not freely pass through the stricture is fluoroscopy needed. In a study of more than 300 patients using wire-guided bougienage, only 8% of the patients required fluoroscopically guided dilation.16
Controlled radial expansion (CRE) balloons are a more recent development in balloon dilation. Individual dilating balloon catheters have three different stepwise inflation diameters that achieve gradated dilation. An in vitro study showed that CRE balloons deliver a consistently reproducible and progressively greater dilating force.17 The three dilation steps are 1 to 1.5 mm apart for all of the CRE balloon sizes. For example, the 6-mm or 18-Fr balloon achieves 12-Fr size at the first inflation step, 15-Fr size at the second step, and 18-Fr at the final dilation step. The 18-mm balloon has three steps at 16 mm, 17 mm, and 18 mm (48-Fr, 51-Fr, and 54-Fr).
Advantages for TTS balloons are that dilation can be performed immediately during the endoscopy and that the endoscope can be passed through the stricture after dilation. Complete endoscopic examination with biopsy and cytology is facilitated. The major disadvantage of balloons is that they are more expensive, and some are fragile. In bougienage, dilation is accomplished by the radial vector of an axially directed force. In contrast, balloon dilators deliver the entire dilating force radially and simultaneously over the entire length of the stenosis, rather than progressively from the proximal to the distal extent. There is less longitudinal shear stress with balloons.18 For balloons, the radial vector force is that exerted by the circumference of the balloon, and the magnitude of this force is related to the length and curvature of the balloon waist at the onset of dilation. The dilating force is greater if the stricture is tighter and longer.19
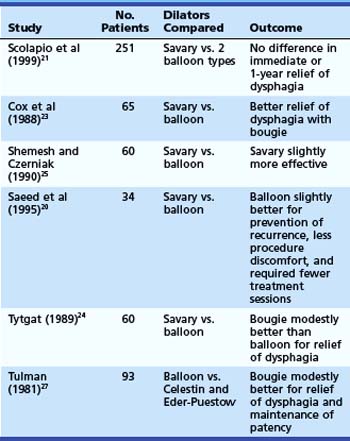
Relatively few patients have been studied in randomized trials comparing the efficacy and safety of the different dilator types.20–27 Of six randomized controlled studies (Table 17.3) comparing wire-guided bougienage with balloon dilation, four concluded that a wire-guided bougie was modestly better than a balloon for reduction of dysphagia; one study found that balloon dilation was modestly better than bougienage for prevention of recurrence, required fewer sessions, and had less procedural discomfort; and one study found no difference with regard to relief of dysphagia or the need for repeat dilation. There seems to be no clear superiority in these various outcome measures for one technique over another. One study comparing the risk of perforation with Maloney dilators, wire-guided bougienage, and balloons concluded that Maloney dilation had a greater risk of perforation than the other two techniques.22 Most endoscopy units have both balloon and wire-guided bougie devices available.
Esophageal Dilation Technique
Patient preparation for esophageal dilation should include holding warfarin or correcting coagulation defects before the procedure. Transient bacteremia is common with endoscopic procedures,28 but routine antibiotic prophylaxis is not recommended as per the 2008 American Society for Gastrointestinal Endoscopy (ASGE) guidelines.29
There is no clear consensus on the optimal size to which a peptic stricture should be dilated. Dysphagia seems to occur when the esophagus is narrowed to less than 13 mm (<39-Fr). Most series report dilation to gauge diameters between 40-Fr and 60-Fr with good relief of symptoms and very low complication rates.30,31 Although no study has documented a higher perforation risk with larger dilator sizes, it is generally assumed that little therapeutic benefit exists with dilation greater than 50-Fr to 54-Fr, and the possibility of increased risk exists.
When dilating a stricture with bougies, the initial dilator size chosen should approximately equal the estimated stricture diameter. It has been recommended that the stepwise increase in bougie size should be not more than three sizes above that at which significant resistance is felt—the “rule of threes.” There are no studies to validate that adherence to this rule increases safety of the procedure. Reported series of patients treated by balloon dilation often use balloon diameters that are larger than the “rule of threes.” Also, in a large series of more than 400 patients in which multiple dilators or a single large dilator (>45-Fr) was passed in a single session, only one perforation was observed.32 Given the risks of perforation, however, it is prudent not to try to accomplish too much dilation in a single setting. Patients can be brought back in 1 to 2 weeks, or even shorter intervals of several days, for repeat sessions to achieve an adequate dilation.
Complications of Esophageal Dilation
The major complications of esophageal dilation are perforation and bleeding. Although there is considerable variation in the studies available, the overall serious complication rate seems to be 0.5%, with perforation and bleeding approximately equal in frequency.33,34 It has been suggested that “blind” Maloney dilation has a higher perforation rate than wire-guided bougie.22 However, if wire-guided techniques are reserved for tighter, longer, more difficult strictures, they may show more complications. Perforation usually is obvious with the patient exhibiting distress and in pain. Subcutaneous emphysema may not develop quickly. A chest x-ray and water-soluble x-ray contrast swallow examination should be performed if perforation is suspected. Surgical consultation is mandatory, although many confined perforations have been managed conservatively with no oral intake and intravenous antibiotics. Bleeding severe enough to require transfusions often leads to a repeat endoscopy to determine if endoscopic therapy is required, although most bleeding from esophageal tears stops spontaneously.
Antibiotic Prophylaxis
In 2008, the ASGE published a consensus guideline for antibiotic prophylaxis before endoscopy.29 The recommendations state that routine antibiotic prophylaxis before endoscopic dilation is not indicated regardless of underlying cardiovascular pathology. Although three prospective trials showed the rate of transient bacteremia after esophageal dilation to be 12% to 22%,35–37 activities of daily living are associated with higher rates of bacteremia. Chewing food is associated with a rate of bacteremia of 7% to 51%, and brushing and flossing the teeth are associated with rates of 20% to 40%.38 The guidelines state that transient bacteremia is not a marker of persistent infection or infective endocarditis and that antibiotic prophylaxis before endoscopic dilation is not recommended.29
Stricture Recurrence
Before PPIs were available, approximately 60% of patients required repeat dilations for recurrent dysphagia.39,40 With PPI therapy, 30% of patients with peptic strictures require repeat dilations within 1 year.41 Factors that predict stricture recurrence depend on the type of stricture. One study evaluated 87 outpatients with either peptic or nonpeptic strictures undergoing initial dilation and followed them for 1 year. In multivariate analysis, narrower stricture diameter and nonpeptic strictures were predictors for early recurrence. The significant predictors for peptic stricture recurrence were the presence of a hiatal hernia and the persistence of heartburn after dilation.42 The technique of self-bougienage can be taught to patients who require very frequent esophageal dilation despite intensive medical therapy and for whom surgery is either contraindicated or unacceptable. Published data on self-bougienage are very limited, but it seems to be both safe and effective.43 Given the efficacy of PPIs, self-bougienage for peptic strictures may be primarily of historical interest.
Recalcitrant Esophageal Strictures
Refractory strictures are more commonly due to corrosive injury or surgical anastomoses than a peptic etiology.44–46 Steroid injection has been recommended for refractory benign esophageal strictures.47–53 Using a 22-gauge or 23-gauge sclerotherapy needle, four aliquots of 0.50 mL of triamcinolone acetonide are injected before dilation. The dilution of triamcinolone injectant can range from 10 to 40 mg/mL for a total dose of 40 to 100 mg of triamcinolone injected. Some studies report injecting proximal to the strictured segment in addition to within the strictured segment, whereas others report injecting into the strictured segment alone. Although most studies perform the injection before dilation, some experts believe that injection after dilation, once the stricture has been disrupted, is more effective. A study of 14 patients with corrosive strictures showed a marked decrease in the dilation requirement compared with their own historical control.53
Similar success was reported in a series of 31 patients with strictures resulting from various causes, including 12 patients with peptic etiology and 8 postsurgical anastomotic strictures, radiation therapy–induced strictures, pill-induced strictures, and sclerotherapy-induced strictures. Intralesional steroid injection led to a significant reduction in the number of dilation sessions in all subjects.52 There has been one prospective, randomized, double-blind, placebo-controlled trial of endoscopic steroid injection for recalcitrant esophageal peptic strictures. In this study of 30 patients, 15 patients received sham therapy followed by TTS balloon dilation and were compared with another 15 patients who received endoscopic steroid injection with subsequent TTS balloon dilation. The groups were identical in pretreatment dysphagia frequency and severity, stricture length and location, use of PPIs, and presence of a hiatal hernia. Although the study was powered to only 60% given the lower than anticipated enrollment, only 2 of 15 patients in the steroid group compared with 9 of 15 patients in the sham group required repeat dilation at 1-year follow-up.47 This prospective randomized controlled trial further supports prior noncontrolled case series advocating the use of steroid injection for recalcitrant strictures.48–53
Removing staples and suture material from the anastomosis using a grasping forceps is thought to reduce stricture recurrence. Another endoscopic treatment that has been reported in the treatment of benign anastomotic esophageal stenosis is the use of electrocautery.54,55 Approximately six radial incisions, each about 2 to 3 mm long, are made using a needle-knife sphincterotome. Hordijk and colleagues55 enrolled 20 patients for treatment with electrocautery who had failed prior dilation therapy for anastomotic esophageal strictures. Of 12 patients with a stricture length of less than 1 cm, all remained symptom-free at 1 year. In eight patients with a stricture length of 1.5 to 5 cm, dysphagia recurred, and a mean of three treatments were necessary. Only two of these eight patients were treatment failures. The use of electrocautery has also been described in combination with balloon dilation56 and argon plasma coagulation.57 At this time, electrocautery should be considered an alternative treatment in short-segment (<1 cm) esophageal, anastomotic ringlike strictures. Whether this modality should be used as a solitary treatment or in conjunction with other modalities has not been determined.58
One novel approach to the treatment of benign, recalcitrant esophageal strictures was the use of endoscissors to cut the stenosed segment. Although this method was reported only as a case report and has not been studied in a randomized trial, endoscissors may be a device to use in refractory cases.59
Surgical treatment of refractory strictures is another alternative. There are two major approaches: (1) antireflux surgery with intraoperative stricture dilation and (2) esophageal reconstruction such as a gastric pull-through or colonic interposition. For peptic strictures that are associated with esophageal shortening, a lengthening procedure such as a Collis gastroplasty may be needed in addition to the antireflux surgery. Comparisons of surgical treatment for peptic strictures by antireflux surgery and intraoperative dilation versus nonsurgical therapy showed similar success rates.60,61 The major advantage to surgery is the decreased need for long-term medical therapy. However, with long-term follow-up, most patients have reinstituted antireflux medications regularly.62 One difficult subset of patients with esophageal peptic stricture comprises patients with esophageal motility disorders such as scleroderma. The abnormal esophageal motor function and mechanical obstruction caused by fundoplication can result in significant postoperative dysphagia, although there are reports of scleroderma patients with excellent surgical outcomes.63 For severe strictures, often not resulting from peptic etiology, surgical resection and reconstruction is required with substantially higher morbidity and mortality.
The use of self-expanding metallic stents (SEMS) for the treatment of refractory benign esophageal strictures has been reported.64 Early success rates are high, although late complications, primarily stricture above the stents, have been reported.65,66 When stents are placed completely within the esophageal lumen, stricture formation also occurs at the distal end of the stent. SEMS generally are contraindicated for the treatment of benign strictures because of difficult long-term complications.
Self-expanding plastic stents (SEPS) have been studied for the treatment of benign esophageal strictures. The only current SEPS that is commercially available is the Polyflex stent, which is a silicone device with an encapsulated monofilament braid made of polyester. An initial positive result with a Polyflex stent for benign esophageal strictures was reported by Repici and colleagues67 in a study in which 12 of 15 patients had long-term relief of dysphagia at follow-up with only 1 patient experiencing stent migration. Several subsequent studies noted, however, that stent migration is common, the patient’s dysphagia regularly returns, and there are serious complications associated with stent placement and removal. A review article by Siersema68
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree