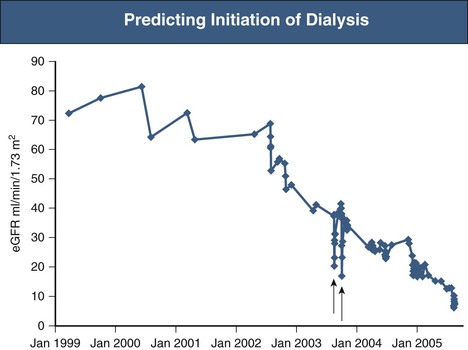
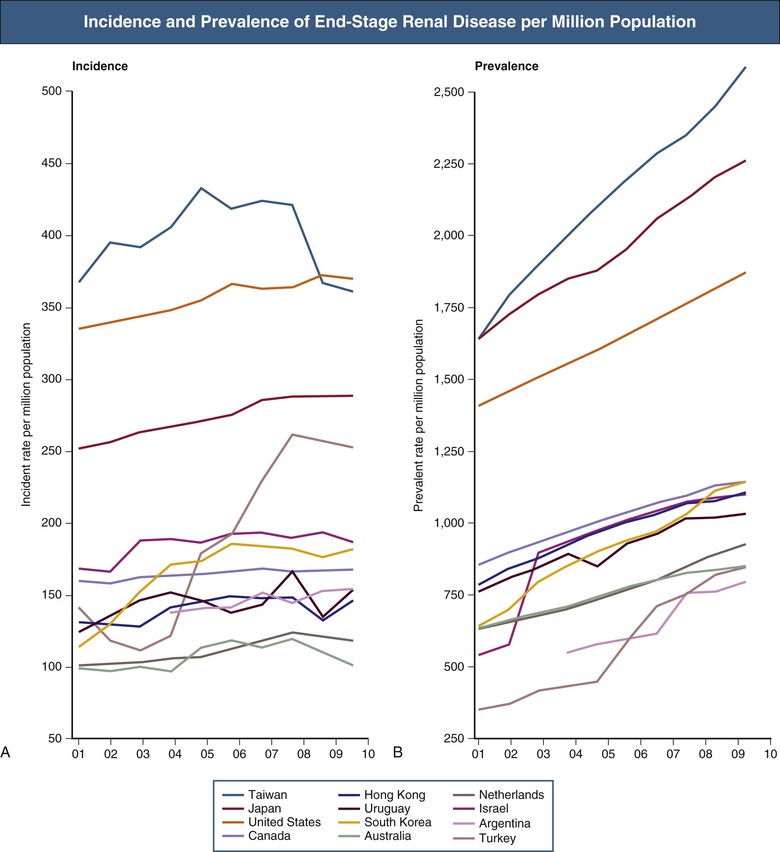
Hugh C. Rayner, Enyu Imai The number of patients receiving renal replacement therapy (RRT) each year, measured as a rate per million population, varies enormously among countries (Fig. 90-1). These rates have risen steadily over the last decade, although this has slowed more recently in some countries. The RRT incidence rate is the product of a complex mix of factors: the incidence and prevalence of diseases that may lead to end-stage renal disease (ESRD), most notably diabetes mellitus1; the effectiveness of chronic kidney disease (CKD) management to slow progression to ESRD; the level of kidney function at which RRT is commenced; and, last, the availability of resources to provide RRT. Renal replacement therapy is a major undertaking for any patient. It is costly and time-consuming and once started may continue for many years. Each ESRD treatment option requires preparation, both physical and psychological. All patients likely to reach ESRD, and their families and care givers, need education about their future options in a form that they find accessible. This chapter sets out an approach to these challenging issues. Preparation for ESRD treatment requires two things: (1) identification of those patients who are at high risk of reaching ESRD and (2) prediction of the likely time when RRT may be needed. Diabetes, heavy proteinuria, declining estimated glomerular filtration rate (eGFR) and previous episodes of acute kidney injury make it more likely that a patient will progress to ESRD. Prediction of ESRD is made easier by a graphic display of eGFRs, derived from creatinine by the Modification of Diet in Renal Disease (MDRD) or Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula, or from cystatin C.2,3 The graph tells the story of a patient’s CKD and is much easier to understand than columns of figures (Fig. 90-2). The trajectory of the eGFR accurately reflects changes in the true GFR over time.4 Advanced CKD care aims to address a number of issues: preservation of remaining kidney function; prevention or treatment of complications of CKD; involvement of the patient and his or her family and care givers in making an informed choice among peritoneal dialysis (PD), hemodialysis (HD), and conservative kidney management; creation of dialysis access in good time; and, in appropriate patients, preparation for kidney transplantation, ideally before dialysis is started. Patients need time, often months, to understand and make decisions about dialysis and its implications.5 The best approach is to transfer patients with declining GFR to a multidisciplinary team at least 12 months before the predicted date of dialysis. Unfortunately, it is often difficult to predict the future trajectory of eGFR.6 eGFR can remain between 20 and 30 ml/min/1.73 m2 for many years, particularly in elderly patients, and so discussions about dialysis may be premature and cause unnecessary anxiety. Conversely, intercurrent illness can cause a sudden drop in GFR and precipitate urgent dialysis. Symptoms and complications of CKD become more common and severe as eGFR falls below 20 ml/min/1.73 m2. Patients with more stable eGFR may be transferred to multidisciplinary care when this threshold is crossed. Advanced CKD care is best delivered by a multidisciplinary team including a dietician, nurse educator, pharmacist, physical therapist, occupational therapist, social worker, and sometimes a trained peer-support volunteer.7 Patients receiving this additional care have better biochemical results, are more likely to start dialysis in a planned way with less hospitalization, and may even have improved survival rates once they have started dialysis.8,9 In addition to being good clinical practice, these programs make good financial sense because the savings in inpatient costs outweigh the costs required to run the clinics. Lack of access to effective predialysis care is a significant issue in the United States. Forty-two percent of new ESRD patients in 2011 had not seen a nephrologist before beginning therapy, and this is associated with a reduction in use of arteriovenous (AV) fistulas as vascular access.60 Among these patients, only 10.5% began hemodialysis with a mature fistula compared with 50.2% of those who had received more than 1 year of nephrologist care. Predialysis programs approach the choices in ESRD care through shared decision making. Whereas clinicians are expert in the technical aspects of dialysis and transplantation, the patient knows best about his or her own needs and preferences. Patients with advanced CKD may not gain sufficient knowledge and understanding to make good decisions from conventional office consultations, even when they have been seen on multiple occasions by a nephrologist.10 Patient education should follow the principles of adult learning: first, assess the patient’s existing level of knowledge and understanding; second, build on this knowledge by the delivery of appropriate information in an appropriate form; and third, establish that the patient has understood and accepted the information given. Education can be delivered both individually and in groups. The clinician is encouraged to send a personal letter to the patient summarizing the issues because this enhances the educational value of consultations. This should be copied to the patient’s family doctor to enhance coordination of care. In a group education session, patients may learn more from fellow patients within a support group than from the group’s facilitator. Furthermore, patient support groups help patients and their relatives appreciate that they are not alone in facing the demands of ESRD. Representatives of all members of the multidisciplinary team, both medical and nonmedical, should deliver the predialysis program. For example, a controlled trial in California studied the value of social worker input to the predialysis program in reducing unemployment.11 In the intervention group, patients and their relatives met regularly with a licensed social worker both before and after starting dialysis, to explore strategies for continuing the patient’s current employment. Blue-collar workers in the intervention group were 2.8 times more likely to continue working. Patients in work had a better quality of life, greater self-esteem, and a more positive attitude toward work. Because it is difficult for dialysis patients to regain jobs once they are lost, this result is particularly valuable for the long-term rehabilitation of patients. In addition to engaging in face-to-face sessions, patients should be directed to the wide range of educational materials available. A number of books have been written specifically for dialysis patients. Many national organizations provide web-based patient information and produce printed and audiovisual material—for example, the National Kidney Foundation in the United States (www.kidney.org). Decision aids can help patients think through their options and choose the treatment that best meets their needs and priorities. For those who are suitable, transplantation offers the best prospect for improved survival and quality of life, especially in younger patients.12 Even in older patients with greater comorbidity, transplantation can improve survival and be cost-effective.13 The options of transplanting kidneys from a deceased or living donor should be discussed, as well as combined kidney and pancreas transplants for people with diabetes. Although outcome data from the local transplanting centers should be made available, published data can be used to inform patients (www.ustransplant.org/annual_reports/current/survival_rates.htm). The ideal time for the transplant to be performed is before dialysis is ever begun: preemptive transplantation. A U.S. study14 showed that preemptive transplantation was associated with a 52% reduction in the risk of graft failure during the first year after transplantation, an 82% reduction during the second year, and an 86% reduction during subsequent years, compared with transplantation after dialysis. Increasing duration of dialysis was associated with increasing odds of rejection within 6 months of transplantation, possibly because of immunologic stimulation during long-term dialysis. The eGFR at the start of dialysis has steadily increased over recent years and varies among countries. Mean eGFR of Japanese patients at the start of dialysis increased from 5.0 ml/min/1.73 m2 in 1989 to 6.5 ml/min/1.73 m2 in 2007. In the United Kingdom, the mean eGFR at the start of dialysis increased from 6 ml/min/1.73 m2 in 1997 to 8.5 ml/min/1.73 m2 in 2012 (www.renalreg.com, UK Renal Registry 2011 Report). In the United States, 17.7% of patients started dialysis with an eGFR of 10 to 15 ml/min/1.73 m2 in 2000; by 2011 this had increased to 29%, with a further 16% starting with an eGFR of more than 15 ml/min/1.73 m2.60 Aging of the population of CKD patients may contribute to earlier initiation of dialysis. In Japan the median age at initiation of dialysis increased from 59 to 69 years from 1989 to 2007.15 A higher-threshold eGFR may be used in diabetics, as they tend to tolerate uremia poorly and are frequently troubled by sodium retention and fluid overload. Other measurements to be considered include a rising serum phosphate, falling serum bicarbonate, and protein-energy malnutrition that persists despite vigorous attempts to optimize dietary intake. A fall in serum albumin is a late sign of reduced protein intake and debility.7 Arbitrary guidelines have been established to gain Medicare approval for dialysis reimbursement in the United States. These include eGFR below 15 ml/min/1.73 m2 for patients older than 18 years and below 20 ml/min/1.73 m2 (Schwartz formula) for those younger than 18 years. Alternatively, adults should have a serum creatinine level above 8 mg/dl (700 µmol/l; >6 mg/dl [530 µmol/l] in diabetes). Patients may also qualify for Medicare if they do not meet these criteria but have uremic symptoms (nausea, vomiting, pericardial pain, acidosis, or hyperkalemia) or pulmonary edema refractory to diuretics. Waiting for patients to develop uremic symptoms carries the risk that the patient will start dialysis in a malnourished state with an increased risk of mortality. Renal failure itself is a catabolic state, and it is commonly difficult for patients on dialysis to regain lost weight. Given the chronic nature of renal disease, patients frequently remain unaware of the severity of their illness. Protein intake may fall spontaneously with the result that symptoms of uremia do not develop, but this is at the expense of a loss of lean body mass. Similarly, patients may gradually reduce their activities as their exercise tolerance declines. It is only when dialysis is started that many patients appreciate how ill they have become. Lack of awareness can be avoided by carefully questioning the patient for insidious symptoms of uremia. For example, patients should be asked to compare their current eating habits and lifestyle with those 6 to 12 months previously. Close friends and relatives provide a useful third-party view of the patient’s well-being. Routine early initiation of dialysis would need to confer significant benefits to justify the added inconvenience to the patient, the additional risk of dialysis-related complications, and the additional cost. Because dialysis treatment has a finite life, either from loss of peritoneal function or failure of vascular access, starting treatment earlier will bring forward the time when further procedures or a change of modality is necessary. Moreover, there is likely to be resistance from many patients to the suggestion that they should start dialysis when they have no symptoms of uremia. The nephrologist would need complete confidence in the laboratory values as well as in the evidence supporting early commencement of dialysis to persuade a reluctant, asymptomatic patient. Starting dialysis is the first step in a lifelong commitment to renal replacement therapy. Patients will be asked to comply with a wide variety of inconvenient and sometimes unpleasant treatments. A high level of compliance is required for a successful outcome, and, particularly in the United States, there is concern about the level of noncompliance that is associated with increased mortality.16 The commitment to dialysis is likely to be greater if the patient feels better after it has started. A prospective study in The Netherlands provides useful data to help the patient and nephrologist agree when to commence treatment.17 Patients who started dialysis with less residual renal function (5 ml/min/1.73 m2 vs. 7 ml/min/1.73 m2) had a poorer quality of life in the early period after starting dialysis. However, this difference was no longer present by the end of the first 12 months of treatment. This has subsequently been confirmed in a randomized controlled trial that showed no difference in survival or quality of life between patients who started dialysis at an eGFR (MDRD equation) of 9.0 ml/min/1.73 m2 and those who started 6 months later at an eGFR of 7.2 (Fig. 90-3).18 Starting dialysis earlier was not associated with better quality of life and incurred higher health care costs.19 One advantage in the early-start group was that a higher proportion of patients who had chosen PD actually started dialysis with PD (80% vs. 70%, P = .01).20 The majority of patients with ESRD are suitable for treatment with either PD or HD. It is difficult to envisage an ethically acceptable trial in which patients are allocated randomly to PD or HD, and the various possible modifications within each modality make a simple comparative trial impractical. Retrospective and prospective nonrandomized comparative studies have failed to indicate a consistent survival advantage for either modality.20 There is some evidence that PD may be inferior to HD over the longer term in patients with coronary heart disease and congestive heart failure.21,22 This contradicts a commonly expressed opinion that PD is gentler for such patients because it avoids rapid fluid shifts and causes less stress on the heart. Change in treatment from PD to HD is associated with an increased risk of hospitalization and mortality.23 A planned change from PD to HD may not be associated with this increased risk, although this has not been studied systematically in a large population. Patients having PD for longer than 7 years are at an increased risk of encapsulating peritoneal sclerosis.24 This may prompt a planned change to HD. There are a few situations in which PD is contraindicated (Table 90-1). Relative contraindications to PD are discussed in the following sections. Table 90-1 Contraindications to dialysis modalities. Absolute and relative contraindications to hemodialysis and peritoneal dialysis. (Adapted from reference 25.) Patients with prosthetic aortic grafts have been successfully treated with PD. HD is usually used initially for up to 16 weeks to allow the graft to be covered with epithelium and so avoid the risk of graft infection via peritoneal dialysate. However, this risk must be balanced against that of bacterial seeding from the patient’s HD access. Body size can be a problem at both ends of the spectrum. Small patients may be intolerant of the volume of dialysate needed to achieve adequate dialysis, particularly if they have negligible residual renal function. Alternative methods of fluid exchange such as nocturnal automated PD can be used to overcome this limitation. It may also be difficult to achieve adequate clearances in patients with a body mass index exceeding 35 kg/m2. Discomfort resulting from increased intra-abdominal volume can be significant in patients with chronic respiratory disease, low-back pain, or large polycystic kidneys. In general, it is hard to predict a patient’s tolerance of intra-abdominal fluid, and so these limitations usually appear after a patient has started PD. The presence of ischemic bowel disease, inflammatory bowel disease, or diverticulitis is likely to increase the incidence of peritonitis as a result of organisms passing through the bowel wall into the peritoneum. Abdominal wall infection may lead to peritonitis via the exit site and catheter tunnel. Screening for methicillin-resistant Staphylococcus aureus (MRSA) before all elective surgical procedures is good practice. Nasal carriage of S. aureus increases the risk of subsequent staphylococcal exit site infection and peritonitis, and clearance of nasal S. aureus with topical mupirocin cream has been shown to significantly reduce the risk of staphylococcal infection at the exit site.26 Patients should ideally commence PD in an adequate nutritional state. Severe malnutrition may lead to poor wound healing and to leakage from the catheter tunnel. In addition, peritoneal protein losses during dialysis may exacerbate hypoalbuminemia. At the other end of the spectrum, it may prove difficult to satisfactorily place a peritoneal catheter through the abdominal wall in patients with morbid obesity. Thereafter, absorption of glucose from the dialysate, which may average as much as 800 kcal/day, may contribute to further weight gain. Contraindications to HD are few (see Table 90-1). As discussed in Chapter 91, access to the circulation can usually be obtained, even in patients with extensive vascular disease or previous surgery. An aversion to needle puncture of the AV fistula is common in the early stages but can usually be overcome by careful use of local anesthetic and nursing encouragement. Severe coagulopathy may make management of anticoagulation for the extracorporeal circuit difficult.
Approach to Renal Replacement Therapy
Treatment Options for Renal Replacement Therapy
Prediction of the Start of Dialysis
Multidisciplinary Care in Advanced Chronic Kidney Disease
Predialysis Education Programs
Education About Transplantation
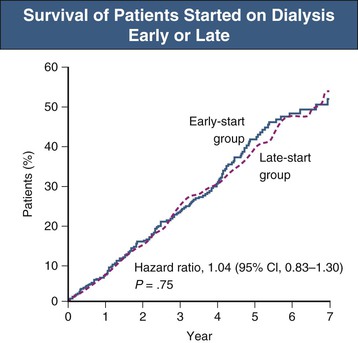
When Should Dialysis Be Started?
Limitations of a Purely Clinical Approach to the Initiation of Dialysis
Limitations of a Purely “Lab Result–Based” Approach in the Initiation of Dialysis
Choice Between Peritoneal Dialysis and Hemodialysis
Contraindications to Peritoneal Dialysis
Contraindications to Dialysis Modalities
Absolute Contraindications
Relative Contraindications
Peritoneal Dialysis
Loss of peritoneal function producing inadequate clearance
Adhesions blocking dialysate flow
Surgically uncorrectable abdominal hernia
Abdominal wall stoma
Diaphragmatic fluid leak
Inability to perform exchanges in absence of suitable assistant
Recent abdominal aortic graft
Ventriculoperitoneal shunt
Intolerance of intra-abdominal fluid
Large muscle mass
Morbid obesity
Severe malnutrition
Skin infection
Inflammatory bowel disease
Hemodialysis
No vascular access possible
Difficult vascular access
Needle phobia
Advanced cardiac failure
Coagulopathy
Fresh Intra-abdominal Foreign Body
Body Size Limitations and Intolerance of Intra-abdominal Fluid Volume
Bowel Disease and Other Sources of Infection
Severe Malnutrition or Morbid Obesity
Contraindications to Hemodialysis
Approach to Renal Replacement Therapy
Chapter 90
Figure 90-1 Incidence (A) and prevalence (B) of end-stage renal disease per million population. All rates are unadjusted. Data from Argentina (2005 to 2007), Japan, and Taiwan are dialysis only. *The data reported here have been supplied by the United States Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the U.S. government. (From reference 60.)