COAGULATION IN DIALYSIS PATIENTS
There remains no universally accepted algorithm for anticoagulation in dialysis patients. This may be due in part to individual patient factors that influence abnormal hemostasis in end-stage kidney disease (ESKD). Although the uremic state tends to cause a bleeding diathesis, dialysis patients have been shown to exhibit hypercoagulable tendencies as well. Some evidence of a thrombotic susceptibility in patients with ESKD includes increased plasma concentrations of fibrinogen (1–3), increased factor VII activity (2,4), and increased fibrinolytic activity (1,3,5). Pulmonary emboli are more common in dialysis patients versus age-matched controls (6), and the anticoagulant proteins C and S have been shown to be deficient in this population (3,7,8).
In addition to patient factors, dialysis procedures themselves invariably induce nonphysiologic levels of turbulent blood flows with high shear stress, which can activate platelets. The exposure of blood to the artificial surfaces of dialysis tubing has also been shown to be thrombogenic, especially in the arterial and venous bubble traps where blood flow can be slower or even static at times (9).
The type of membrane selected for the dialysis procedure may impact thrombogenicity. Several studies have shown that membranes composed of regenerated cellulose (cuprophane) lead to more activation of coagulation compared with membranes composed of newer materials such as polyacrylonitrile or polysulfone (10–13). The results of two studies suggest that polysulfone membranes have minimal effects on coagulation activation as measured by thrombin–antithrombin complex levels and platelet activation (10,14). The process of dialysis membrane sterilization may affect anticoagulation. This was suggested after significant thrombocytopenia was noted following the introduction of dialyzers that has been sterilized using electron beam technology (15).
The presence of an arteriovenous (AV) access is yet another predisposing factor toward turbulent blood flow, platelet activation, and thrombosis (7). The risk for access thrombosis is greater in polytetrafluoroethylene (PTFE) grafts compared to native AV fistulae (16), and conditions such as systemic lupus erythematosus (SLE) may exacerbate the tendency to thrombose the access (17).
With the interplay of all of the aforementioned clinical entities, adequate anticoagulation should be viewed as essential to the hemodialysis procedure.
 UNFRACTIONATED HEPARIN
UNFRACTIONATED HEPARIN
The anionic glycosaminoglycans present in unfractionated heparin prevent clotting of blood by the inhibition of serine proteases (18). Because heparin used for hemodialysis is a mixture of components, heparin formulations are standardized in terms of international units (IU), using a United States Pharmacopeia (USP) standard heparin as a reference. The therapeutic window for adequate anticoagulation without excessive bleeding using heparin is narrow. As such, measurement of the anticoagulant effects of heparin would be optimal. Unfortunately, the measurement of blood heparin levels is not practical. Historically, assays such as the activated clotting time (ACT) and the whole blood partial thromboplastin time (WBPTT) have been used to measure the anticoagulability of heparin. Both these tests use whole blood and can produce results in minutes, making them desirable for clinical use. Following enactment of the Clinical Laboratory Improvement Amendments (CLIA) of 1988, automated clotting time measurement systems replaced the manually performed WBPTT as the means of monitoring anticoagulation during hemodialysis in the United States (19). This law requires that any laboratory testing of human specimens be performed in a certified laboratory that incorporates quality control, proficiency testing, and calibration verification in its testing program.
Such procedures are associated with increased overhead costs and are difficult to incorporate into everyday use in a busy hemodialysis center. Indeed, many dialysis units in the United States have abandoned the testing of blood for clotting parameters and moved toward developing heparin-dosing algorithms. One such strategy has been developed based on a nonlinear mixed effects population kinetic model that was designed to estimate heparin-loading doses and infusion rates (20,21). In this equation, the loading dose of heparin is derived as follows (EQUATION 4.1):
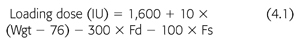
In this equation, Wgt is the patient’s weight (kg), Fd indicates the presence (Fd = 1) or absence (Fd = 0) of diabetes, and Fs indicates that the patient is (Fs = 1) or is not (Fs = 0) a smoker. The proposed infusion rate based on this model was 1,750 IU/h. Using these parameters to predict heparin-loading doses and infusion rates, one study noted a significant improvement in dialyzer reuse rates with no decrease in the delivered dose of dialysis (19).
Data from Ward et al. (22) demonstrate that the sensitivity to heparin in nonuremic hemodialysis patients correlates with body weight. Low et al. (23) suggested an initial heparin-loading dose of 20 to 25 IU/kg was sufficient to maintain adequate anticoagulation during dialysis based on measured WBPTT. In 2002, the European Renal Association (24) published guidelines for anticoagulation in hemodialysis patients. Their recommendations include a loading dose of approximately 50 IU/kg followed by a continuous infusion rate of 800 to 1,500 IU/h. This approach of giving a loading dose followed by a continuous infusion of heparin offers the nephrologist the ability to titrate dosing to the lowest possible amount to prevent clotting of the circuit while avoiding excessive bleeding risk. The heparin infusion is then titrated off over the last 30 minutes of the dialysis session to allow for clotting time to decrease and to avoid bleeding when the access needles are withdrawn.
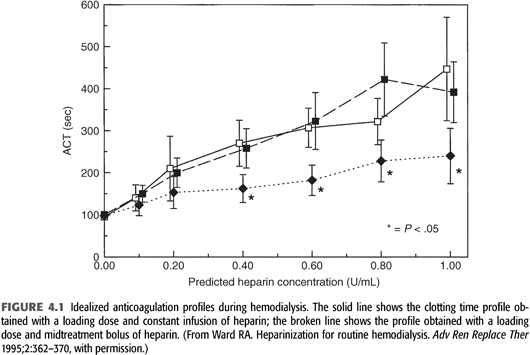
An alternative anticoagulation strategy uses intermittent heparin dosing. An initial loading dose of heparin is administered, followed by one or more additional bolus doses during dialysis. Intermittent dosing is simple and eliminates the need for an infusion pump and syringe. However, this approach results in periods of over- and under-anticoagulation compared to the loading dose and constant infusion method (25). Furthermore, the use of intermittent dosing is time-consuming and requires the constant attention of dialysis personnel to ensure that timely heparin boluses are given. FIGURE 4.1 illustrates the anticoagulation profiles obtained with a continuous infusion versus intermittent bolus dosing of heparin after the initial loading dose is given.
Invariably, nephrologists will encounter patients who are at a high risk for bleeding complications. These patients include, but are not limited to, those who have recently had or will be undergoing surgery, those with a history of gastrointestinal bleeding, those with a history of hemorrhagic stroke, and those with severe liver disease. In these cases, low-dose heparin use has been shown to be an effective strategy for anticoagulation (26–28). Low-dose heparinization is achieved using a smaller bolus dose of 10 to 25 IU/kg. Maintenance heparin can then be given either as an infusion at a rate of 11 to 22 IU/kg/h or through small intermittent boluses. However, with low doses of heparin, increases in clotting time have been observed to vary by ±50% from day to day in individual patients who are given the same dose of heparin (29). Taking this into account, low-dose heparin should be reserved for select cases and performed with the understanding that minimizing the bleeding risk may result in increased clotting of the extracorporeal circuit on some occasions.
In addition to the inherent bleeding risks associated with its use, heparin therapy has other associated side effects including worsening of osteoporosis and dyslipidemia, allergic reactions such as pruritus, and thrombocytopenia. Hyperkalemia may be seen due to inhibition of aldosterone synthase (30), although this is seldom an issue for patients with ESKD. The development of heparin-induced thrombocytopenia (HIT), a rare but life-threatening immune-mediated disorder, requires absolute heparin avoidance during dialysis.
 LOW MOLECULAR WEIGHT HEPARIN
LOW MOLECULAR WEIGHT HEPARIN
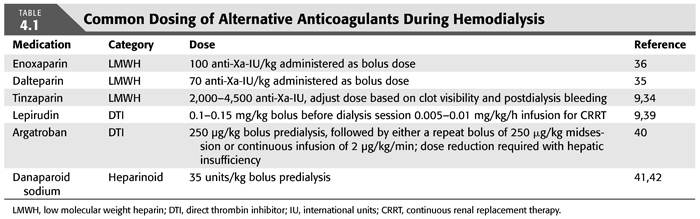
LMWH preparations are made from depolymerized fractions of heparin. These smaller saccharide chains have a molecular weight of 4 to 8 kDa and inhibit factor Xa up to threefold more when compared to unfractionated heparin, with less affinity for thrombin inhibition (9). For these reasons, monitoring the effect of LMWH requires the measurement of antifactor Xa activity as the activated partial thromboplastin time (aPTT) and ACT are not reliable (31,32). LMWH preparations have been extensively studied during dialysis in patients with ESKD. The longer half-life of LMWH allows these preparations to be given as a single predialysis dose, usually based on body weight (33–36). However, should the dialysis session be scheduled for greater than 4 hours, additional LMWH may need to be given either as a bolus or through continuous infusion (37,38). TABLE 4.1 illustrates the commonly used dosages of LMWH during hemodialysis.
Some authors advocate the use of LMWH as the preferred agent for anticoagulation in patients with ESKD (24). The appeal of single bolus dosing is one potential advantage offered compared to unfractionated heparin. Indeed, in one study, scanning electron microscopy was used to demonstrate that membrane-associated clotting during dialysis was less after treatment with LMWH compared to unfractionated heparin (43). Among the other purported benefits of LMWH include a reduction in the need for blood transfusions (44) and less of a tendency toward hyperkalemia (45).
There has been some debate in the literature about the effect of LMWH on the lipid profiles of dialysis patients. Several studies have demonstrated improvement in the total cholesterol (46–51) and triglyceride levels (46,49–51) in dialysis patients after switching from unfractionated heparin to LMWH. These studies included both diabetic and nondiabetic subjects, and the period of study ranged from 6 months to 4 years. Another study examined the effect of LMWH on lipid profiles in prevalent dialysis patients who had been previously receiving unfractionated heparin. Over 1 year, improvements were noted in total cholesterol, triglyceride, and Apo B concentrations. After the first 12 months, patients were randomly chosen to either continue with LMWH or revert to unfractionated heparin. Although the improvement in lipid profiles continued in the LMWH group, the unfractionated heparin group sustained no further benefit (52).
In contrast, a multicenter trial examining 153 patients on LMWH and 153 patients on unfractionated heparin failed to demonstrate any significant differences in lipid profiles between the two groups except for higher high-density lipoprotein (HDL) cholesterol levels in the heparin group (53). A smaller study by the same authors showed an improvement in lipid profiles after switching 24 incident dialysis patients from LMWH to unfractionated heparin after the first 6 months of treatment (54). These latter two studies suggest that it may be premature to assume that a benefit in lipid profiles is assured when using LMWH.
One factor that has precluded the routine use of LMWH during dialysis procedures is the concern for excess bleeding in patients with kidney disease. A meta-analysis investigating the safety of LWMH in patients with ESKD found no significant difference in bleeding events or extracorporeal circuit clotting when compared to unfractionated heparin (55). Other studies, however, have raised concerns. Brophy et al. (56) used the measurement of thrombin generation time in a prospective ex vivo study to demonstrate that the anticoagulant effect of enoxaparin was enhanced in patients with ESKD. These data suggested that even at similar levels of antifactor Xa activity, the effect of enoxaparin in ESKD remains unpredictable and could subject patients with ESKD to bleeding complications. Other clinically significant events such as retroperitoneal hemorrhage, gastrointestinal bleeding, intracranial bleeding, and hemorrhagic pericardial effusion have been described in patients with chronic kidney disease (CKD) stages 4 or 5 who were treated with fixed doses of LMWH (57). Additional concern when using LMWH preparations is that they are only partially reversible using protamine. The package inserts of the three major LMWH preparations commonly used in the United States (enoxaparin, dalteparin, and tinzaparin) state that these agents should be used with caution in patients with a creatinine clearance less than 30 mL/min. These facts, combined with the lack of readily available antifactor Xa level monitoring, suggest that patient safety concerns may outweigh the other purported advantages of using LMWH for chronic dialysis procedures.
 ALTERNATIVE ANTICOAGULATION STRATEGIES
ALTERNATIVE ANTICOAGULATION STRATEGIES
Perhaps no other clinical diagnosis has focused more attention on the need for alternative anticoagulant agents than HIT. HIT is a life-threatening disorder resulting from antibodies formed against complexes of platelet factor 4 (PF4) and heparin (39). HIT has been classified into type I and type II, with type II being the most clinically significant entity. In type II HIT, platelet counts usually decline by 40% to 50% from baseline approximately 5 to 10 days after heparin exposure. Clotting of both the venous and arterial circulation can be seen, with 50% of patients developing clot formation of some kind within 30 days on average (58). Both unfractionated and LMWH have been shown to cause HIT, although the association with LMWH appears to be smaller.
The frequency of HIT in the population with ESKD has been reported to range anywhere from 0% to 12% (39,58). If the diagnosis of HIT is suspected, antibody testing should be used for confirmation, given the potential life-long implications and cost associated with treatment. Treatment of HIT includes absolute heparin avoidance, including all heparin flushes, ointments, and catheter lock solutions. In addition to these precautions, systemic anticoagulation is recommended in patients with HIT to prevent thrombotic complications.
Some alternative anticoagulation strategies that can be used in clinical situations such as HIT as well as in patients who are at high risk for bleeding will now be discussed. TABLE 4.1 provides the usual dosing schemes for many of these agents during hemodialysis.
Direct Thrombin Inhibitors
Direct thrombin inhibitors prevent thrombin generation by binding to the active site on the thrombin molecule, thereby blocking the conversion of soluble fibrinogen to insoluble fibrin. The three direct thrombin inhibitors approved for use by the U.S. Food and Drug Administration (FDA) are recombinant hirudin, argatroban, and bivalirudin.
A natural derivative from the saliva of the Hirudo medicinalis leech species, hirudin, was actually the first anticoagulant used for dialysis procedures in the early 1920s before giving way to heparin (59). The primary route of excretion of hirudin is through kidney elimination. Therefore, its half-life is markedly prolonged in patients with kidney disease. Lepirudin, a form of recombinant hirudin, has been studied in patients with kidney disease including patients undergoing CRRT (60,61). Especially in cases of anuria, the dosing requirements for hirudin are small (60); however, meticulous monitoring of the aPTT levels should be undertaken. In patients on CRRT, lepirudin can be dosed through continuous infusion at 0.005 to 0.01 mg/kg/h, whereas for chronic dialysis, a bolus dose of 0.15 mg/kg can be given before the session (39). Dosing should be carefully adjusted to achieve an aPTT of 1.5 to 2 times normal without exceeding 100 seconds in order to minimize bleeding risk. The reader should also keep in mind that the aPTT does not increase linearly with lepirudin blood levels (62). Therefore, aPTT times that are elevated above the goal range may reflect excessively high levels of blood lepirudin. These situations are all the more dangerous considering that there is no specific antidote to lepirudin. The formation of antihirudin antibodies that can occur in up to 40% of patients who receive lepirudin for more than 5 days and may enhance its anticoagulant effect is another factor to consider when using this agent (63).
Argatroban is a synthetic derivative of L-arginine that was first studied during hemodialysis procedures in the mid-1980s (64). The appeal of this agent for use in patients with kidney disease stems from the fact that it is metabolized and secreted by the liver. A retrospective review of 47 patients with HIT showed that argatroban was a safe and effective anticoagulant for both chronic dialysis and CRRT (40). In a prospective, randomized, three-way crossover study in patients with prevalent ESKD, argatroban was shown to be safe and effective for chronic dialysis (65). Of the three argatroban dosing strategies used in this study, there were no significant differences in achieved urea reduction ratio, thrombotic, or bleeding events. The clearance of argatroban through the dialysis membrane was also found to be insignificant. Using the methods employed in this study, a 250 μg/kg bolus of argatroban with a repeat 250 μg/kg bolus given midway through the session should achieve adequate anticoagulation. An alternative strategy would be to use an initial bolus of 250 μg/kg of argatroban followed by a continuous infusion of 2 μg/kg/min. Hospitalized patients who are already receiving an argatroban infusion for other reasons can be safely dialyzed without interruption of therapy. Given the hepatic metabolism of argatroban, liver function testing is imperative before using this agent. In cases of hepatic insufficiency, the dosing of argatroban should be reduced to 0.5 μg/kg/min through continuous infusion, and careful monitoring of the aPTT should be performed (39). In general, the aPTT has been found to correlate well with plasma argatroban levels (66), and the goal of therapy should be to achieve an aPTT of 1.5 to 3 times normal. The concern of antibody production against argatroban has not been reported.
Bivalirudin is a semisynthetic 20 amino acid derivative of hirudin. Its primary mode of elimination is through intracellular hydrolysis, with only 20% urinary excretion (67). Similar to lepirudin, the half-life of bivalirudin is prolonged in patients with impaired kidney function. With normal kidney function, the elimination half-life is 25 minutes, and is prolonged to 3.5 hours in dialysis patients (67). Although promising as an alternative anticoagulant to treat HIT, there are little published data regarding the use of bivalirudin in dialysis patients. Currently, the FDA has approved this agent only for use in conjunction with percutaneous coronary intervention.
Danaparoid
Danaparoid sodium is a heparinoid composed primarily of the nonheparin glycosaminoglycan heparan sulfate. Danaparoid exerts its anticoagulant effect by inhibition of factor Xa. Although not currently on the market in the United States, there is considerable experience treating HIT patients with danaparoid in Canada, Europe, and Australia. Danaparoid is considered to be a safe alternative agent to use in patients with HIT despite a 3.2% rate of cross-reactivity with heparin (68). A survey of 81 dialysis units in the United Kingdom revealed that 36% of patients with ESKD who tested positive for HIT were treated using danaparoid (69). Danaparoid is reported to allow successful dialysis without clotting or bleeding complications when administered as a single predialysis dose of 35 U/kg (41,42). Given that kidney excretion is the primary route of danaparoid elimination, monitoring of antifactor Xa levels should be performed before each dialysis session with a goal level of 0.5 to 0.8 IU/mL. Even with dose adjustment, these patients must be considered at risk for bleeding complications.
Regional Citrate Anticoagulation
Regional anticoagulation with citrate has been demonstrated to be an effective alternative for use of heparin. Trisodium citrate infusion into the blood entering the dialyzer chelates calcium and prevents coagulation. Anticoagulation is reversed, and ionized calcium levels restored, through a combination of citrate loss into the dialysate and infusion of calcium into the venous blood line or calcium influx from the dialysate. Multiple protocols have been described for citrate anticoagulation (70–73). The simplest method involves infusion of concentrated trisodium citrate solution into the arterial blood line, coupled with the use of dialysate containing normal levels of calcium and magnesium (71,73). The citrate infusion rate is adjusted to provide a 25% to 75% increase in ACT at the entry to the dialyzer (71–73). Other methods use calcium- and magnesium-free dialysate and reinfuse calcium into the venous blood line (70,74). A prospective study in dialysis patients who required heparin free treatment after recent surgery demonstrated that regional citrate anticoagulation was superior to the use of a heparin-coated polyacrylonitrile membrane in terms of clotting phenomena that necessitated termination of the dialysis session (75). Citrate anticoagulation may be useful in patients with heparin allergy, but problems with hypocalcemia, hypernatremia, and metabolic alkalosis render this technique more cumbersome than routine heparin anticoagulation (76,77). The dialysate bicarbonate concentration should be reduced to 25 to 30 mM to guard against metabolic alkalosis, which may develop if the bicarbonate generated from citrate metabolism is added to the normal influx of bicarbonate from the dialysate (78). In extreme cases, failure to adequately correct ionized calcium levels has been reported to result in electrolyte imbalance and cardiac arrest (79), and an ability to monitor ionized calcium concentrations during hemodialysis is considered essential for patient safety. One large but uncontrolled trial reported a 99.6% success rate using a regional citrate anticoagulation protocol in 1,009 high-flux dialysis procedures in 59 outpatients with ESKD (80). However, for the abovementioned safety concerns, regional anticoagulation using a citrate protocol is typically reserved for hospitalized patients who are at high risk for bleeding complications. Regional citrate anticoagulation by itself is insufficient to treat patients with HIT given the need for systemic anticoagulation.
Oral Anticoagulation
In general, oral anticoagulation is a poor choice for treating patients with ESKD. There are ample data that suggest warfarin may enhance the process of vascular calcification (81). Furthermore, no studies have conclusively demonstrated a benefit of warfarin on clotting profiles during hemodialysis. In studies looking at both tunneled, cuffed dialysis catheters (82) and PTFE grafts (83), minidose warfarin was no better than placebo at maintaining access patency. As expected, the rates of adverse bleeding events were higher in patients treated with warfarin. Another smaller study used a randomized, crossover design to investigate the efficacy of LMWH versus no anticoagulation in 10 patients with ESKD treated with warfarin [international normalized ratio (INR) 2.0–3.0]. The patients who received no additional anticoagulation had significantly greater clotting of the dialyzer when compared to the LMWH group (84). Taken as a whole, these data suggest that oral warfarin should be reserved for those patients with ESKD who have a defined hypercoagulable state or for those with other compelling indications for continuous anticoagulation such as a mechanical heart valve. A recent retrospective cohort study of incident hemodialysis patients with atrial fibrillation actually suggested an increased risk for new stroke in patients taking warfarin (85). These data seem to be in accordance with some of the more recent studies recognizing advanced chronic kidney disease and ESKD as risk factors for increased bleeding when using oral anticoagulation. In particular, the Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, Labile international normalized ration, Eldery (>65 years), Drugs/alcohol concomitantly (HAS-BLED) scoring system defines an increased risk of 1 year major bleeding events in anyone with a history of kidney transplant, ESKD, or a serum creatinine value of greater than 2.6 mg/dL receiving oral anticoagulation for atrial fibrillation (86).
Antiplatelet Agents
The Dialysis Access Consortium Study Group has investigated the use of antiplatelet agents specifically to enhance the patency and reduce the failure rate of the arteriovenous access in hemodialysis patients. One study found that clopidogrel had a modest, but statistically significant, effect in reducing the rate of fistula thrombosis at 6 weeks after access creation (87). Unfortunately, clopidogrel therapy did not result in a significant increase in the proportion of fistulas that were suitable for dialysis compared to placebo (defined as the ability to sustain a blood pump speed of 300 mL/min or greater in 8 out of 12 dialysis treatments). Another randomized controlled trial examined the effect of dipyridamole plus aspirin given twice daily on the ability to maintain patency in newly created arteriovenous grafts (88). In this study, the treatment group exhibited a modest but significant increase in the incidence of primary unassisted graft patency at 1 year. In both studies, treatment with antiplatelet therapy did not result in significant adverse events, including major bleeding, compared to placebo.
Hemodialysis without Anticoagulation
Dialysis procedures can be successfully performed without the use of anticoagulation. This may be necessary in patients with an active bleeding diathesis (e.g., intracranial hemorrhage). In such cases, membranes with the lowest thrombogenicity (e.g., polysulfone) should be used. To minimize the chances for clotting, treatment times should be ideally limited to 2 to 3 hours and blood flows should be attempted at least as fast as 250 mL/min. Rinsing of the extracorporeal circuit with 25 to 150 mL of saline through the arterial port is another technique, which can aid in the prevention of circuit clotting (89,90).
 FACTORS PREVENTING ADEQUATE ANTICOAGULATION
FACTORS PREVENTING ADEQUATE ANTICOAGULATION
Both dialyzer clotting and excessive bleeding can occur in patients with ESKD who have apparently stable heparin prescriptions. These situations can usually be traced to either patient-related issues or technical problems in therapy delivery.
Patient-Related Issues
Comorbid conditions such as infections, intercurrent illnesses, or underlying disease exacerbations [systemic lupus erythematosus (SLE), chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF)] may change a patient’s coagulation status. Intercurrent illnesses have been shown to affect the elimination rate of heparin (91). In cases of infection or disease exacerbations, vigilance should be maintained to ensure proper anticoagulation is maintained. These conditions often require a temporary increase in the heparin dosage to prevent extracorporeal circuit clotting.
Heparin can physically interact with a number of drugs (92); however, with the possible exception of nitroglycerin (93,94), there is little evidence that drugs alter the anticoagulant effect of heparin. Smoking increases the elimination rate of heparin (95), and a history of smoking was found to be a significant covariate in population pharmacodynamic models of heparin dosing in hemodialysis patients (21).
Patients treated with erythropoietin require more heparin to prevent dialyzer clotting (96,97). Whether this fact is a reflection of the induction of higher hematocrit concentrations or a result of some reversal of the uremic bleeding tendency is a matter of speculation. In either case, patients receiving erythropoietin-stimulating agents may require higher doses of anticoagulants to achieve successful dialysis.
Technical Problems in Therapy Delivery
As summarized in TABLE 4.2, there are many factors that can potentially go wrong during any given hemodialysis session, thereby leading to either dialyzer clotting or excessive bleeding. These situations should be investigated before attributing the problem to a faulty anticoagulation prescription.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


