Richard G. Phelps, A. Neil Turner
Anti–Glomerular Basement Membrane Disease and Goodpasture Disease
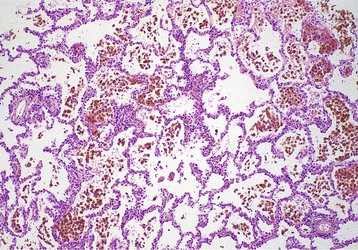
The syndrome of renal failure and lung hemorrhage was associated with the name of Ernest Goodpasture by Stanton and Tange in their description of nine cases in 1958.1,2 All nine patients presented with lung hemorrhage and acute renal failure and died within hours or days. These features had been prominent in the case of a young man who died during the influenza pandemic of 1919, whose postmortem findings were memorably reported by Goodpasture1: “The lungs gave the impression of having been injected with blood through the bronchi so that all the air spaces were filled” (Fig. 24-1).
Several diseases are now recognized as being associated with alveolar hemorrhage and rapidly progressive glomerulonephritis (RPGN). Nevertheless, this remains a striking clinical entity with relatively few causes and few pathogenetic mechanisms.
Because the first recognized mechanism was anti–glomerular basement membrane (anti-GBM) antibody formation and deposition, Goodpasture’s name is firmly associated with anti-GBM disease (Goodpasture disease), even though this is responsible for only a proportion of patients with Goodpasture syndrome of lung hemorrhage and RPGN. The terminology used in this chapter is defined in Table 24-1.
Table 24-1
Definition of terms associated with anti-GBM disease and Goodpasture syndrome.
RPGN, Rapidly progressive glomerulonephritis.
| Anti–Glomerular Basement Membrane (GBM) Terminology | ||
| Term | Definition | Pathogenesis |
| Pulmonary renal syndrome | Renal and respiratory failure | Many causes (see Fig. 24-7) |
| Goodpasture syndrome | RPGN and alveolar hemorrhage | Several causes (see Box 24-2) |
| Anti-GBM disease | Disease associated with antibodies specific for (any) components of GBM | Most important are Goodpasture disease and Alport syndrome post-transplant anti-GBM disease |
| Goodpasture disease | Disease associated with autoantibodies specific for α3(IV)NC1 May include RPGN, lung hemorrhage, or both | Autoimmunity to α3(IV)NC1 |
| Alport syndrome post-transplant anti-GBM disease | Glomerulonephritis associated with anti-GBM antibodies developing after renal transplantation in Alport syndrome patients | Immunity to foreign collagen IV chains not expressed in Alport syndrome patients usually α3 or α5 (IV)NC1 |
Etiology and Pathogenesis
Autoimmunity to a Component of Glomerular Basement Membrane
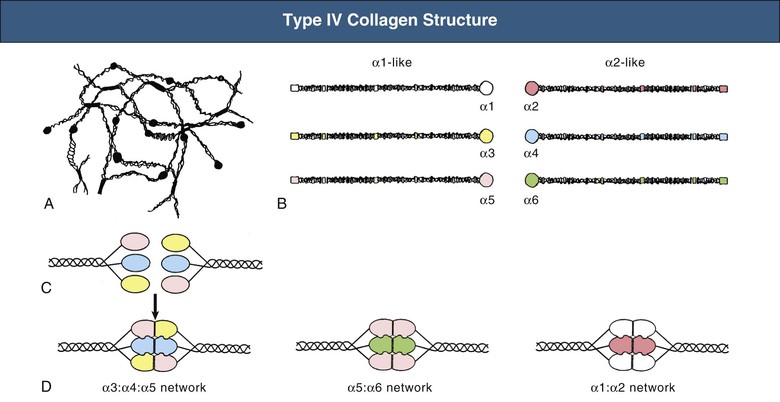
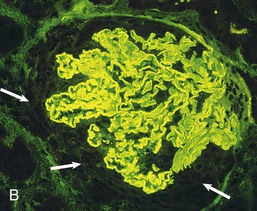
Goodpasture disease is caused by autoimmunity to the carboxyl terminal, noncollagenous (NC1) domain of a type IV collagen chain, α3(IV)NC1, also known as the Goodpasture antigen3,4 (Fig. 24-2). Type IV collagen is an essential constituent of all basement membranes. In most tissues, it is composed of trimers comprising two α1 chains and one α2 chain, but there are also four tissue-specific chains, α3 through α6.5,6 Three of these, α3 through α5, are found in GBM as well as in the basement membranes of the alveolus, the cochlea, parts of the eye (including corneal basement membrane and Bruch membrane), the choroid plexus of the brain, and some endocrine organs.
All patients with RPGN, lung hemorrhage, and anti-GBM antibodies have antibodies to α3(IV)NC1, usually binding predominantly to a single or a very restricted set of epitopes. Some patients also have antibodies to other basement membrane constituents, including other collagen IV chains, usually in low titer.
Predisposing Factors
Both environmental and genetic factors appear to be important in etiology. There are strong associations between Goodpasture disease and HLA class II alleles, including DRB1*1501 and DR4 alleles, whereas DR1 and DR7 confer strong and dominant protection.7 Of likely increasing importance, it is now clear that treatment of multiple sclerosis (MS) with alemtuzumab, a monoclonal antibody targeting CD52 on B and T cells, is associated with the development of new autoimmunity, including anti-GBM disease, sometimes as late as 4 years after treatment. Only a handful of cases have been reported during development of the drug for MS, but with wider use, this could become a numerically very significant predisposing factor. It remains to be established whether carriage of DRB1*1501, which is overrepresented in MS, influences the risk of developing anti-GBM disease after alemtuzumab therapy.
Precipitating Factors
The onset of acute disease has long been linked to various precipitating factors. Theories of pathogenesis include factors that alter antigen processing to generate peptides that are usually destroyed or hidden, and to which tolerance is therefore deficient8,9 and molecular mimicry.10 None of these is proved. Reports of temporal and geographic clustering of cases suggest an environmental trigger,11 but no specific infectious agent has been consistently identified. Hydrocarbon exposure has been linked to disease onset in several striking case reports; but in some cases, such exposure may simply trigger lung hemorrhage in patients who already have the disease. Furthermore, exposures of this kind are very common in the modern world. Similarly, cigarette smoking may precipitate lung hemorrhage in patients who already have circulating autoantibodies, but there is no evidence for a role in causation.
In several cases, renal trauma or inflammation has preceded the development of the disease (Box 24-1). These may alter α3(IV)NC1 turnover and metabolism qualitatively or quantitatively, providing an opportunity for self-tolerance to be broken. Qualitative changes in the basement membrane epitopes presented to T cells could be a result of overloading of the usual or recruitment of alternative processing pathways, such as extracellular processing by proteases released into inflamed glomeruli. The quantity of α3(IV)NC1 presented to T cells may be greater where there has been damage to the basement membrane, as occurs in systemic small-vessel vasculitis (see Chapter 25). Some features suggest that an anti-GBM response may be a secondary phenomenon in some patients with vasculitis.12,13 The association with membranous nephropathy is interesting because the thickened GBM in that disease contains increased amounts of the tissue-specific type IV collagen chains, including the Goodpasture antigen. The same could apply to a recently suggested possible association with longstanding type 1 diabetes mellitus.14
Mechanisms of Renal Injury
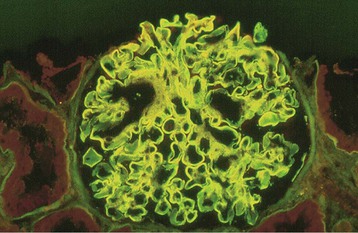
The α3(IV)NC1 autoantibodies are central in the pathogenesis of Goodpasture disease15,16 (Fig. 24-3). Antibodies eluted from the kidneys of patients who had died of Goodpasture disease rapidly bind to the GBM and cause glomerulonephritis (GN) when they are injected into monkeys.17 Furthermore, the deposited antibodies are predominantly IgG1 and are complement fixing. Contributions to renal injury mediated by such antibodies come from complement and from neutrophil and macrophage infiltration. T cells are essential for driving autoantibody production by T cell–dependent B cells, and in experimental renal disease, they are critical in producing glomerular crescents,15,18 which are a usual feature of Goodpasture disease. Moreover, in mice engineered to express the human susceptibility HLA allele, DRB1*1501, α3(IV)NC1-specific CD4 T cells are sufficient to transfer disease between animals.19
Agents that downregulate inflammation by inhibiting interleukin-1 or tumor necrosis factor, or that inhibit recruitment of inflammatory cells by blockade of adhesion molecules or chemoattractants, suppress injury in experimental models of anti-GBM disease. Evidence in humans and in experimental animals supports the severity of renal injury being increased by proinflammatory cytokines or by stimuli likely to elicit them, such as bacteremia.18 Crescent formation is seen in aggressive inflammatory GN, as described in Chapter 16 (see Fig. 16-8).
Lung Hemorrhage
Lung hemorrhage in Goodpasture disease (but not in small-vessel vasculitis, the other major cause of Goodpasture syndrome) occurs only if there is an additional insult to the lung, which is usually cigarette smoke. However, infection, fluid overload, toxicity from inhaled vapors or other irritants, and the systemic effects of some cytokines are also possibilities. This is probably because the alveolar capillary endothelial cell provides a better barrier between circulating immunoglobulin and the underlying basement membrane than the diaphragm-free fenestrations of the glomerular capillary endothelial cell. In the glomerulus, antibodies have direct access to the GBM because of the fenestrations of glomerular endothelium. Other sites at which the Goodpasture antigen is found are not involved in Goodpasture disease, except possibly the choroid plexus, where the endothelium is again fenestrated, and more rarely the eye.
Epidemiology
Goodpasture disease is rare, with a possibly increasing incidence in Caucasian populations approaching 1 case per 1 million population per year.14 The incidence in black and South Asian populations appears to be lower. The incidence in other racial groups is uncertain. There is a slight male predominance. Lung hemorrhage is more common in younger patients.
Clinical Manifestations
Between 50% and 75% of patients present with acute symptoms of lung hemorrhage and are found to be in a state of advanced renal failure. Symptoms are usually confined to the preceding few weeks or months, but rapid progression (during days) or much slower progression (during many months) may occur. A lack of systemic symptoms, other than those related to anemia, is typical, although an apparently minor infection often triggers the clinical presentation.
Lung Hemorrhage
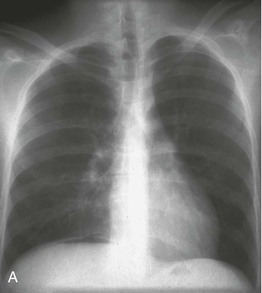
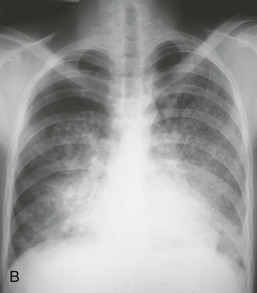
Lung hemorrhage may occur with renal disease or in isolation. Presenting symptoms may include cough and hemoptysis, but hemorrhage into alveolar spaces may result in marked iron deficiency anemia and exertional dyspnea, even in the absence of hemoptysis. Depending on the degree and chronicity of lung hemorrhage, examination findings may include pallor, dry inspiratory crackles, signs of consolidation, or respiratory distress. Recent lung hemorrhage typically is shown on the radiograph as central shadowing that may traverse fissures and give rise to the appearance of an air bronchogram (Fig. 24-4). However, even lung hemorrhage sufficient to reduce the hemoglobin concentration may cause only minor or transient radiographic changes, and these cannot be confidently distinguished radiologically from other causes of alveolar shadowing, notably edema or infection. The most sensitive indicator of recent lung hemorrhage is an increased uptake of inhaled carbon monoxide (Dlco). Patients with lung hemorrhage are usually current cigarette smokers.
In isolated lung disease, progressive alveolar or fibrotic disease or pulmonary hemosiderosis may be suspected, although at least hematuria is usually present. This may continue for months or in rare cases recurrently for years before significant renal disease occurs.
Glomerulonephritis
Patients with GN may notice dark or red urine, but progression to oliguria is sometimes so rapid that this phase, if it occurs, is missed. In one third to half of patients, GN occurs in the absence of lung hemorrhage. In this subgroup, because systemic symptoms are generally not prominent, presentation is often late with renal failure.
Whatever the early pattern of disease, once significant renal impairment has occurred, further deterioration in renal function is usually rapid. Presentation at or shortly after acceleration of the disease process is common, and patients may demonstrate very rapid loss of renal function and life-threatening lung hemorrhage. Urinalysis always reveals hematuria (even in apparently isolated pulmonary disease), usually modest proteinuria, and dysmorphic red blood cells and RBC casts on microscopy. The kidneys are generally of normal size but may be enlarged. Hematuria may be substantial or associated with loin pain in acute disease.
Pathology
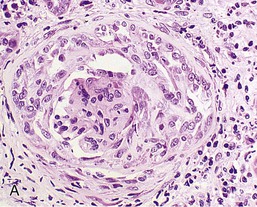
Renal biopsy is essential as it provides diagnostic and prognostic information. Typical appearances are of diffuse proliferative GN with variable degrees of necrosis, crescent formation, glomerulosclerosis, and tubular loss (Fig. 24-5). The degree of crescent formation and tubular loss correlates with renal prognosis. Characteristically, the crescents all appear to be of similar age and cellularity. When biopsy is performed earlier in the disease, changes may be limited to focal and segmental mesangial expansion, with or without necrosis. This progresses to hypercellularity and then to more general changes, including fractures of the GBM and Bowman capsule, neutrophils in the glomeruli, and glomerular capillary thrombosis.20