5
Androgens and Sexuality in Aging Men
Case History
A 64-year-old stockbroker was referred by his family physician for erectile dysfunction (ED), which had been getting worse over a 2-year period. He also complained of decreased libido, which was apparently first noticed after his vasectomy 20 years ago but had gotten worse. On occasions when he was able to just manage sexual penetration, he needed time and effort to achieve ejaculation and would often lose his erections before he could reach climax. His erections on waking from sleep had become infrequent and trivial. He felt frustrated, depressed, irritable, and easily tired, and thought that these might have been caused by the considerable work-related stresses because of recent fluctuations in the stock market. His sleep had been disturbed, and he recalled waking up struggling for breath. All these caused appreciable strain on his marital relationship of 30 years. Twelve months ago he had been prescribed an antidepressant in addition to paracetamol-codeine (acetaminophen-codeine) tablets for chronic low back pain. Since having coronary angioplasty with stenting 4 years ago, he had not had further episodes of angina, and he was taking daily low-dose aspirin, ramipril, atenolol, and simvastatin for secondary prevention. He was overweight and was advised on diet and exercise for his non-insulin-dependent diabetes. He was not a cigarette smoker and consumed some alcohol only on social occasions.
Apart from abdominal adiposity, the general physical examination was unremarkable. Blood pressure was 150/100mm Hg, and neurovascular assessment of his lower limbs did not reveal any significant abnormality. He was well androgenized, and testes were equal in size (20 mL) and normal in consistency. There was no clinical abnormality in the penis. Blood biochemistry showed the following results: glycated Hb 7.2% (6.0–7.0 for good glycemic control), total cholesterol 5.4mmol/L (<5.5), triglycerides 3.2mmol/L (<1.8), high-density lipoprotein (HDL) cholesterol 1.0mmol/L (>0.9), and low-density lipoprotein (LDL) cholesterol 2.9mmol/L (<3.5). Liver functions, urea, creatinine, and electrolytes were within normal limits. Hormonal evaluation showed a plasma thyroid-stimulating hormone (TSH) level of 1.5mU/L (0.3–4.3), prolactin 270mU/L (<340), total testosterone 9.5nmol/L (10–35), luteinizing hormone (LH) 3 U/L (2–9), and follicle-stimulating hormone (FSH) 15 U/L (1–6).
Decline in Blood Androgen Levels in Aging Men
There has been criticism concerning the use of the term andropause to denote the state of age-related decrease of plasma testosterone (T) level in some elderly men.1 Although andropause may indeed be a misnomer for a condition associated with a decline in the plasma testosterone level, in contrast to the term menopause for women going through the physiological transition signaling an almost complete cessation of ovarian function, it does provide, especially for the layman, a catchy term that depicts the predicament men are in.
Androgens comprise mainly testosterone (T) and include dihydrotestosterone (DHT), dehydroepiandrosterone (DHEA), androstenedione, and androstenediol, although, strictly speaking, DHEA is not a true androgen as it does not bind androgen receptors.2 Sixty percent of T is bound to sex hormone-binding globulins (SHBG), whereas the bioavailable T (BT) consists of T bound to albumin (38%) and free T (FT) (2%).3 There is an age-related decline in plasma T levels,4 and this decline in the plasma FT and BT levels is even more significant and consistent.4,5
A single decreased plasma T result is euphemistically called hypotestosteronemia and has no clinical value unless it can be confirmed by one or more properly conducted and repeated tests. Standardized data on age-specific normal reference ranges for different ethnic, cultural, socioeconomic, and geographical groups are yet to be established. Currently, interpretation of plasma T levels in elderly men is based on a normal reference range provided by available values in young men.6,7 Consensus has yet to be reached regarding whether total T, FT, BT, or calculated FT should be the measurement of preference.6,8 Availability and accuracy of the tests selected or used are also matters requiring consideration.
The Physiological Effects of Androgens on Sexual Function in Men
Genetic sex is established when fertilization occurs. After 6 weeks of gestation, genetically directed sex differentiation commences with the formation of the testes from the mesonephric or wolffian duct. Initially, the process does not appear to be androgen-dependent. However, subsequent differentiation into the epididymides, vasa deferentia, and seminal vesicles requires the presence of androgens. At about 8 weeks of gestation, the fetal Leydig cells begin to secrete T, and the levels peak at 11 to 18 weeks. Conversion of T to DHT by 5α-reductase occurs at 13 weeks, and DHT promotes the masculinization of the external genital primordia.9
Male sexuality is the expression of the interaction between androgens and behavior. In humans, hormonal influences on behavior are much less potent than in animals, and the effects of social and cultural factors can be considerable. At puberty when the testes begin to secrete androgens, sex drive, sexual interest, sexual performance, and copulatory ability increase. The hypothalamus, the hippocampus, the medial preoptic area, and the limbic system become activated as the production of androgens rises.10
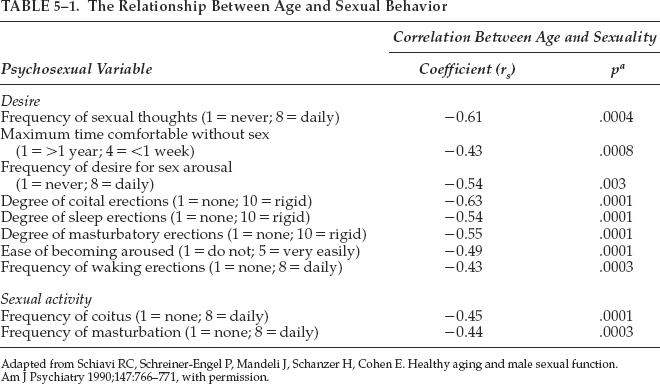
Shiavi et al11–13 reported in 1990–1993 their investigation of factors that contributed to health, well-being, and marital satisfaction in a study of 77 healthy men aged 45 to 74 years in stable sexual relationships. There was a significant negative correlation between age and sexual desire, arousal, and activity, as well as a significant age-related increase in the prevalence of erectile dysfunction (Table 5–1). Significant age-related decreases in frequency, duration, and degree of nocturnal penile tumescence were also reported. Prospective and retrospective data on the prevalence of sexual dysfunction were found to be unrelated to hormonal variables.
In another study involving 57 young controls, 50 healthy potent older controls, and 267 men with erectile dysfunction, Korenman et al14 found no difference in the plasma T and BT levels in men with and without erectile dysfunction, when these levels were adjusted for age and body mass index (Fig. 5–1). Hypogonadal BT levels were found to be associated with LH levels within the normal range, indicating hypothalamic-pituitary dysfunction. There was also correlation between low basal LH levels and hyporesponsiveness to gonadotropin release testing. These findings suggest that secondary hypogonadism and erectile dysfunction are in fact two independent conditions prevalent in older men.
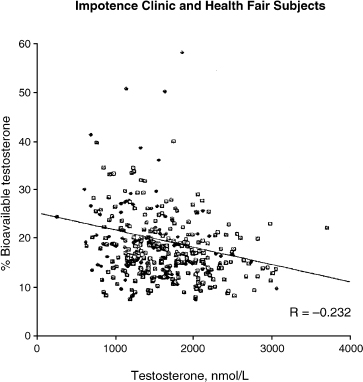
FIGURE 5-1. Distribution of bioavailable versus total testosterone in erectile dysfunction patients. (Adapted from Korenman SG, Morley JE, Mooradian AD, et al. Secondary hypogonadism in older man: its relation to impotence. J Clin Endocrinol Metab 1990;71:963–969, with permission.)
Using the International Index of Erectile Function (IIEF), Rhoden et al15 showed that, although there was a clear association between erectile dysfunction and age, no correlation was observed between the occurrence of or the severity of erectile dysfunction and total T levels. There is thus no evidence that changes in plasma T levels contribute to erectile dysfunction in healthy aging men.
Although there are wide interpersonal variations in the age-related decline in plasma T and FT levels,4 a decline of androgen-related sexual behavior in individual subjects was shown to be reproducible at certain critical plasma T levels in a study involving testosterone-treated hypogonadal patients and tamoxifen-treated eugonadal men.16 In the same study, there was no evidence that androgen administration in excess of the individually determined critical levels would enhance sexual function.
The effect of T on sexual function is mainly centrally mediated through libido, which is regulated partly by T-dependent psychic factors. Testosterone influences the frequency of sexual thoughts and behavior.11 However, only the very low end of normal range of testosterone is needed for normal libido and sexual performance.11
In the studies of Carani et al17,18 using the Rigiscan to examine nocturnal penile tumescence and erectile response to visual erotic stimuli, the number of satisfactory nocturnal penile tumescence responses, in terms of both circumference increase and rigidity, was less in hypogonadal men than in normal controls. This was significantly increased by androgen replacement. However, such difference in erectile response and improvement with androgen replacement were not observed when the hypogonadal men and normal control were exposed to visual erotic stimuli. These findings suggest that nocturnal erections are T-dependent, but not the erections in response to visual erotic stimuli.
Testosterone has been reported to modulate the expression of nitric oxide synthase in the corpus cavernosum and the production of nitric oxide.19,20 It acts on the cavernosal arterioles enhancing penile rigidity,17 and influences, probably peripherally rather than directly through cognitive behavior, genital sensitivity and pleasurable enhancement of erectile activity.21
Knowledge regarding the effects of androgens on sexuality has come from research in other primates, observations of clinical outcome of castration in men, and studies of androgen replacement in hypogonadal men. T replacement and supplementation in hypogonadism increases the frequency of sexual fantasy, sexual arousal, desire and activity, nocturnal erections, ejaculation, and orgasm.10 However, in a study examining the relationship between sexual function and pharmacologically manipulated T levels, treatment with T was shown to benefit only men with abnormally low plasma testosterone levels.22 The findings not only indicated that relatively low T levels were needed for erectile function and sexual activity and feelings, but they also helped explain why some mildly hypogonadal men continue to have normal sexual function and why there is a lack of good correlation between sexual function and plasma T levels in the normal range.
In studies involving eugonadal men, administration of T produced an increase in sexual interest, but there was no change in sexual relationship or activity.23–25 In a single-blind placebo-controlled study by Anderson et al24 of 31 men treated with T for male contraception, trough plasma T levels were increased by 80% and peak plasma T levels by 400 to 500% with intramuscular T injections. There were no changes in frequency of sexual intercourse, masturbation, or penile erectile response. Only an increase in interest in sex was reported during treatment with T. The authors concluded that supraphysiological levels of T could promote some aspects of sexual arousability without stimulating sexual activity in eugonadal men in stable heterosexual relationships. This contrasts with the stimulation of sexual behavior in hypogonadal men treated with T.10 Fig. 5–2 demonstrates the effect of testosterone on the Sexuality Experience Scale 2 (Psychosexual Stimulation) scores.
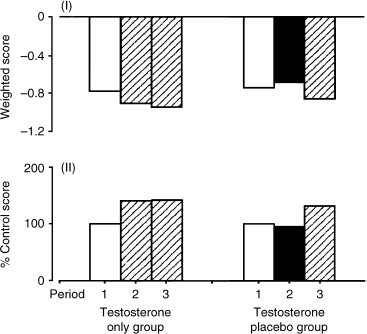
FIGURE 5-2. Effect of administration of testosterone on responses to the Sexuality Experience Scale 2 (Psychosexual Stimulation) scores (a and b). In treatment period 1, no testosterone is administered to either group; in treatment period 2, testosterone is administered to the testosterone-only group and a placebo to the testosterone placebo group; in treatment period 3, testosterone is administered to both groups. (Adapted from Anderson RA, Bancroft J, Wu FC. The effect of exogenous testosterone on sexuality and mood of normal men. J Clin Endocrinol Metab 1992; 75:1505–1507, with permission.)
The Role of Dehydroepiandrosterone (DHEA), Dihydrotestosterone (DHT), and Estrogens in Male Sexual Function
Dehydroepiandrosterone (DHEA) is generally regarded as an androgen, although, as alluded to earlier on,2 it is not a true androgen as it does not bind androgen receptors. In spite of its widespread use in complementary medicine, studies on the role of DHEA in male sexuality are lacking. Anecdotal reports suggest that DHEA may increase libido. In a study of 280 healthy men and women aged 60 to 79 years,26 a small increase in plasma T and estradiol was noted with use of DHEA over 1 year. There was a significant increase in most libido parameters in the elderly women. No potentially harmful accumulation of DHEA sulfate (DHEAS) and active steroids was reported.
Overall, the physiological and pharmacological effects of T are the combined effects of T and those of its androgenic and estrogenic metabolites through respectively, 5α-reductase activity and aromatization.
The effects of dihydrotestosterone (DHT) on male sexual function may be inferred from the gamut of sexual disorders, which may occur in men receiving treatment with 5α-reductase inhibitor. These include decreased libido, erectile dysfunction, and ejaculatory disorders.27
Estradiol (E2) is the main estrogen in men and is produced from the conversion of T under the influence of aromatase in the peripheral adipose tissues. Twenty percent of E2 in men is secreted by the testes and indirectly derived from the adrenals.
Chronic exposure to estrogen and phytoestrogen was found to cause impairment of erectile function in animal studies.28 Raised plasma E2 levels had been reported in men with erectile dysfunction (ED) who were receiving treatment for epilepsy,29 liver cirrhosis, or chronic renal failure.30–32 However, in a study involving 75 consecutive adult male patients with ED, raised plasma E2 level was observed in only one patient (1.3%). These findings seem to suggest that E2 is probably of relevance in ED only in clinical conditions predisposed to hyperestrogenism.33
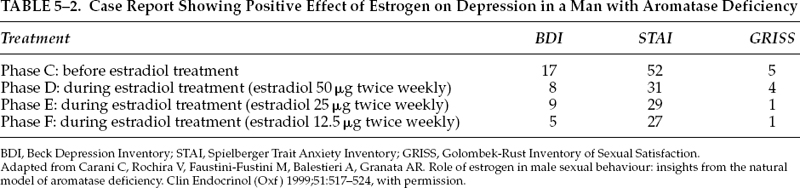
On the other hand, hypoestrogenism in men with aromatase deficiency appeared to be associated with difficulty in sexual functioning although not with problem in gender identity.34 Treatment of hypoestrogenism with E2 led to an increase of libido, erotic fantasy, and frequency of coitus and masturbation.34 Table 5–2 summarizes the positive effect on depression in a patient with aromatase deficiency. The role of estrogen in male sexual function/dysfunction, therefore, still remains speculative and unclear.
Discussion of Case History
The patient’s plasma T level is decreased at 9.5nmol/L (10–35). Other chapters of this book discuss the appropriate approach that should be taken to investigate this and similar results so as to exclude physiological fluctuations and to correctly establish a diagnosis of androgen deficiency. Suffice it to say that, although several factors are present in the patient’s clinical profile that are known to contribute to androgen deficiency and hypogonadism, such as age, stress, depression, antidepressant medication, probable sleep apnea, overweight, and long-term use of narcotic analgesic, the presence of any or all of these factors in association with a single decreased plasma T level does not necessarily suggest androgen deficiency and hypogonadism.
Apart from decreased T, other age-related hormonal changes including declining growth hormone release and hypothyroidism may also contribute to sexual dysfunction. The diagnosis of androgen deficiency and hypogonadism, therefore, has yet to be made.
Given that androgen deficiency, medications, and psychological factors are among the main causes of decreased libido, there was evidently a significant overlay of psychological factors in the patient’s decreased libido, as this was first noticed after his vasectomy, long before other comorbid conditions came into play. It would hence be simplistic and clinically naive to attribute the patient’s sexual problems to just decreased T, even if androgen deficiency were confirmed.
As regards the patient’s retarded ejaculation, psychological stresses related to his strained marital relationship, antidepressant medication, and possible diabetic autonomic neuropathy are probable contributory determinants.
The main reason for the patient’s referral was erectile dysfunction. There is little doubt that vascular factors (coronary artery disease), diabetes mellitus, depression, medications (particularly β-adrenergic blocker atenolol), possible androgen deficiency, stress, and probable obstructive sleep apnea and secondary anxiety had contributed to his inability to obtain and maintain his erections for satisfactory sexual intercourse.
The patient’s history of coronary artery disease was relevant and significant. There has been, in fact, increasing evidence that erectile dysfunction may well be synonymous with endothelial dysfunction, and that erectile dysfunction is likely to be a predictor or harbinger of coronary artery disease.
Forty percent of men with erectile dysfunction with no cardiac symptoms had coronary artery disease diagnosed on investigation with coronary angiography.35 Coronary artery disease was diagnosed in 16% of men with erectile dysfunction compared with 5% of men without erectile dysfunction.36 The severity of erectile dysfunction was found to correlate with the number of occluded coronary vessels in patients with coronary artery disease.37
The management of the patient’s sexual dysfunction (decreased libido, erectile dysfunction, and retarded ejaculation) will involve appropriate laboratory evaluation and consideration of a host of therapeutic options ranging from counseling to pharmacological intervention. The patient should understand and appreciate the value of lifestyle modifications, weight reduction, good control of his diabetes, lipid profile, and cardiovascular risk factors. It will be helpful too for him to be firmly reassured that, other than its possible psychological impact, uneventful vasectomy has no adverse bearing on either sexual function or androgen profile.
This case highlights the complex nature and multifactorial basis of male sexual function and dysfunction and the need for a multidisciplinary approach in its diagnosis and management. Nonpharmacological measures are no less important than the new and sophisticated pharmacotherapeutic options, which have engendered a paradigm shift in its treatment.
Conclusion and Key Points
• There is well-documented and well-recognized age-related decline in androgen levels in men.
• Decreased plasma T level has to be appropriately confirmed and diagnosis of hypogonadism collaborated with clinical symptom-complex of androgen deficiency.
• The physiological and pharmacological effects of T are the combined results of T and its androgenic and estrogenic metabolites.
• The influence of androgens on male sexuality is mainly centrally mediated through libido.
• T may influence erectile function by modulating the expression of nitric oxide synthase and the production of nitric oxide in the penile erectile tissues.
• Relatively low plasma T levels are sufficient for normal erectile function.
• Other hormonal and nonhormonal factors may also affect sexual function.
• Treatment with T would only improve sexual dysfunction in men who are truly hypogonadal.
• It would be simplistic and naive to attribute sexual dysfunction to just decreased plasma T levels, especially if these are the result of opportunistic investigation.
REFERENCES
1. Morales A, Lunenfield B. Investigation, treatment and monitoring of late-onset hypogonadism in males. Aging Male 2002;5:74–86
2. Roger M. DHEA: to have or hav’nt. ISSIR Newsbulletin 2001; 7:14
3. Horton R. Testicular steroid transport, metabolism and effects. In: Becker KL, ed. Principles and Practice of Endocrinology and Metabolism. Philadelphia: JB Lippincott; 1995:1042–1047
4. Vermeulen A. Androgens in the aging male—clinical review 24. J Clin Endocrinol Metab 1991;73:221–224
5. Pearson UJD, Blackman MR, Metter EJ, Waclawiw Z, Carter HB, Harman SM. Effect of age and cigarette smoking on longitudinal changes in androgens and SHBG in health men. Abstracts of the 77th Annual Meeting of the Endocrinological Society 1995; Abstract P2:129.
6. Vermeulen A, Kaufman JM. Diagnosis of hypogonadism in the aging male. Aging Male 2002;5:170–176
7. Beilin J, Chew G, Feddema P, O’Leary P. Testosterone levels in aging male: a population based study utilizing measurement of total and calculated bioavailable testosterone. 54th Annual Meeting & Clinical Laboratory Expo of the American Association of Clinical Chemistry 2002; Presentation No. D-21.
8. Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999;84:3666–3672
9. Quigley CA. The androgen receptor: physiology and pathophysiology In: Nieschlag E, Behre HM, eds. Testosterone— Action, Deficiency, Substitution. 2nd ed. New York: Springer; 1998:33–106
10. Christiansen K. Behavioural correlates of testosterone. In: Nieschlag E, Behre HM, eds. Testosterone—Action, Deficiency, Substitution. 2nd ed. New York: Springer; 1998:106–142.
11. Schiavi RC, Schreiner-Engel P, Mandeli J, Schanzer H, Cohen E. Healthy aging and male sexual function. Am J Psychiatry 1990;147:766–771
12. Schiavi RC, Schreiner-Engel P, White D. Mandeli J. The relationship between pituitary-gonadal function and sexual behaviour in healthy aging men. Psychosom Med 1991;53:363–374
13. Schiavi RC, White D, Mandeli J, Schreiner-Engel P. Hormones and nocturnal penile tumescence in healthy aging men. Arch Sex Behav 1993;22:207–215
14. Korenman SG, Morley JE, Mooradian AD, et al. Secondary hypogonadism in older man: its relation to impotence. J Clin Endocrinol Metab 1990;71:963–969
15. Rhoden EL, Teloken C, Mafessori R, Souto CA. Is there any relation between serum levels of total testosterone and the severity of erectile dysfunction? Int J Impot Res 2002;14:167–171
16. Gooren LJG. Androgen levels and sex functions in testosterone-treated hypogonadal men. Arch Sex Behav 1987; 16:463–473
17. Carani C, Scrteri A, Marrama P, Bancroft J. The effects of testosterone administration and visual erotic stimuli on nocturnbal penile tumescence in normal men. Horm Behav 1990;24: 435–441
18. Carani C, Granata AR, Bancroft J, Marrama P. The effects of testosterone replacement on nocturnal penile tumescence and rigidity and erectile response to visual erotic stimuli in hypogonadal men. Psychoneuroendocrinology 1995;20:743–753
19. Zvara P, Sioufi R, Schipper HM, Begin LR, Brock GB. Nitric oxide mediated erectile activity is a testosterone dependent event: a rat erection model. Int J Impot Res 1995;7:209–219
20. Mills TM, Reilly CM, Lewis RW. Androgens and penile erection: a review. J Androl 1996;17:633–638
21. Davidson JM, Kwan M, Greenleaf WJ. Hormonal replacement and sexuality. Clin Endocrinol Metab 1982;11:599–623
22. Buena F, Swerdloff RS, Steiner BS, et al. Sexual function does not change when serum testosterone levels are pharmacologically varied within the normal male range. Fertil Steril 1993;59:1118–1123
23. O’Carrol R, Bancroft J. Testosterone therapy for low sexual interest and erectile dysfunction in men: a controlled study. Br J Psychiatry 1984;145:146–151
24. Anderson RA, Bancroft J, Wu FC. The effect of exogenous testosterone on sexuality and mood of normal men. J Clin Endocrinol Metab 1992;75:1503–1507
25. Bagatell CJ, Heiman JR, Matsumoto AM, Rivier JE, Bremner WJ. Metabolic and behavioural effects of high-dose exogeneous testosterone in healthy men. J Clin Endocrinol Metab 1994;79:561–567
26. Baulieu EE, Thomas G, Legrain S, et al. Dehydroepiandro-sterone (DHEA), DHEA sulfate, and aging: contribution of the DHEA Study to a socio biomedical issue. Proc Natl Acad Sci USA 2000;97:4279–4284
27. McClellan KJ, Markham A. Finasteride: a review of its use in male pattern hair loss. Drugs 1999;57:111–126
28. Srilatha B, Adaikan PG, Ng SC. Chronic estrogen exposure causes erectile dysfunction. Int J Impot Res 2002;14:S7
29. Murialdo G, Galimberti CA, Fonzi S, et al. Sex hormones and pituitary function in male epileptic patients with altered or normal sexuality. Epilepsia 1995;36:360–365
30. Van Steenbergen W. Alcohol, liver cirrhosis and disorders in sex hormone metabolism. Acta Clin Belg 1993;48:269–283
31. Wang YJ, Wu JC, Le SD, Tsai YT, Lo KJ. Gonadal dysfunction and changes in sex hormones in postnecrotic cirrhotic men: a matched study with alcoholic cirrhotic men. Heptogastroenterology 1991;38:531–534
32. Griffin JE, Wilson JD. Disorders of the testes. In: Fauci AS, et al, eds. Harrison’s Principles of Internal Medicine. 14th ed. New York: McGraw-Hill; 1998:2087–2097
33. Chew KK. Estradiol and Testosterone in male sexual dysfunction. Second Asian ISSAM Meeting on the Aging Male Abstract Book. 2003;S2–03
34. Carani C, Rochira V, Faustini-Fustini M, Balestieri A, Granata AR. Role of estrogen in male sexual behaviour: insights from the natural model of aromatase deficiency. Clin Endocrinol (Oxf) 1999;51:517–524
35. Pritzker MR. The penile stress test: a window to the hearts of man? Circulation 1999;100:711
36. Anderson M, Nicholson B, Louie E, Mulhall JP. An analysis of vasculogenic erectile dysfunction as a potential predictor of occult cardiac disease. J Urol 1998;159(suppl 5):118
37. Greenstein A, Chen J, Miller H, Matzkin H, Villa Y, Braf Z. Does severity of ischaemic coronary disease correlate with erectile function? Int J Impot Res 1997;9:123–126
< div class='tao-gold-member'>