■ Invasive modalities include endoscopic retrograde pancreatography (ERCP) or endoscopic ultrasound (EUS) and are part of the initial workup for lesions of the ampulla.
■ Imaging modalities can provide information on extent of local disease including depth of invasion, if applicable. CT scans are the usual initial diagnostic imaging studies performed, due to ready availability and ease of interpretation.
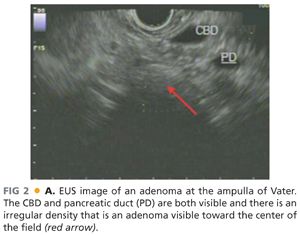
■ CT scan has use for the detection of nodal disease or distant metastases, whereas EUS has a great deal of use in the detection of depth of invasion of ampullary adenoma and hence has a role in the workup of disease amenable to local resection (FIG 2). This point is not without controversy, as others have argued that CT scan is not superior to EUS in terms of nodal staging.
■ When stricture at the ampulla is suspected, ERCP can be used to clarify the extension of the stricture into the pancreatic parenchyma or the distal CBD; this information is critical for operative planning.
■ For strictures that extend beyond the ampulla itself, the operation of choice would be a pancreaticoduodenectomy or CBD reconstruction, that is, hepaticojejunostomy depending on the etiology.
■ ERCP, hepatobiliary iminodiacetic acid (HIDA) scan, and MRCP with secretin stimulation or sphincter manometry may be used to make the diagnosis of SOD.
■ Patients with delayed excretion of contrast after ERCP (>45 minutes), CBD dilatation greater than 1.2 cm, and elevated alkaline phosphatase or serum glutamic oxaloacetic transaminase (SGOT) (greater than two times the upper limit of normal) have excellent response to endoscopic sphincterotomy alone without other diagnostic maneuvers.
■ Patients who meet some but not all of these criteria benefit from further diagnostic testing with manometry, and those patients in whom manometry is abnormal may benefit from sphincterotomy.
■ Patient with biliary pain but without any of the aforementioned abnormalities may undergo manometry but have generally lower rates of success following sphincterotomy even in the presence of abnormal manometry.
■ Although this has not been validated prospectively, one small study suggested that HIDA scan with morphine as an adjunct has the advantage of a noninvasive test that, when positive, correlates well with a positive surgical outcome.10
■ MRCP with secretin stimulation is another modality that has been used to demonstrate SOD dysfunction in patients with prior Roux-en-Y gastric bypass for morbid obesity.8
■ Sphincter manometry is performed via a miniaturized pressure catheter that is placed in the sphincter of Oddi using a side-viewing endoscope. Four categories of manometric abnormalities are defined and include the following:
■ Papillary stenosis, which is defined as persistently elevated resting pressures within the sphincter of Oddi that are unresponsive to pharmacologic means of sphincter relaxation
■ Dyskinesia, in which the response to pharmacologic relaxation is preserved within the context of elevated resting pressures
■ Tachyoddia, in which there is an increased frequency of spontaneous contractions of the sphincter and physiologically abnormal (paradoxical) response to cholecystokinin.11
■ Paradoxical sphincter response, in which an increase in sphincter tone is observed in response to cholecystokinin administration
■ Endoscopy with secretin stimulation test may also be used in the stratification of patients with pancreas divisum who are likely to benefit from surgical sphincteroplasty. Briefly, EUS may be used to visualize the minor pancreatic duct. After administration of secretin, increase in ductal dilatation gives evidence to an obstructive papillary stenosis. The absence of this dilatation should prompt consideration of further trials of nonsurgical therapy.
SURGICAL MANAGEMENT
Preoperative Planning
■ Biliary tract decompression, that is, by percutaneous transhepatic biliary drain placement or ERCP, may be required if the patient has presenting symptoms of biliary obstruction.
■ Preoperative placement of an epidural catheter for pain control is a useful adjunct for a planned open operation and limits postoperative dependence on narcotic pain medication.
■ General strategies to optimize patients for major surgery should be employed as indicated. These may include optimization of nutritional status as well as smoking and alcohol cessation.
Positioning
■ The patient is positioned supine on the operating table. Sequential compression device boots are placed. Unless there is a contraindication to heparin administration, 5,000 units of unfractionated heparin are given in the preoperative holding area as deep venous thrombosis (DVT) prophylaxis.
■ Preoperative antibiotics are administered within 1 hour of incision. Appropriate antibiotics should cover for skin flora as well as for gram-negative enterics and anaerobes.
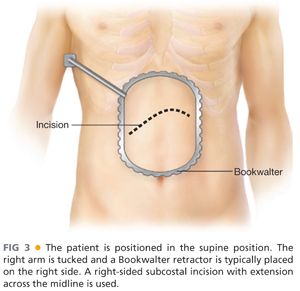
■ Our practice is to extend the left arm and tuck the right arm and place the Bookwalter (or similar) retractor on the patient’s right side. A right subcostal (Kocher) incision with extension a short distance across the midline is used (FIG 3).
TECHNIQUES
INCISION AND EXPOSURE
■ A right subcostal (Kocher) incision is made with extension across the midline to facilitate exposure. The incision should be made approximately two fingerbreadths inferior to the costal margin. Adequate exposure is facilitated by the placement of a fixed retractor such as the Bookwalter or Thompson. If the gallbladder is present, a cholecystectomy is typically performed. We suggest leaving a long cystic duct stump to facilitate placement of transcystic duct stump biliary catheter to guide identification of the ampullary orifice. This maneuver can optimize the location of the subsequent duodenotomy. The liver is retracted superiorly and the hepatic flexure is completely mobilized such that it can be safely retracted inferiorly out of the operative field.
KOCHER MANEUVER
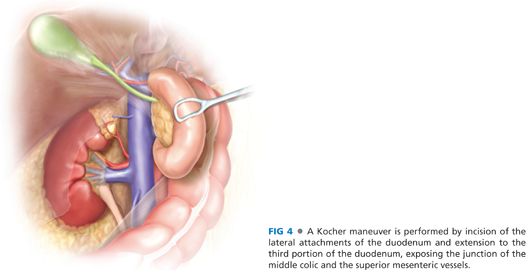
■ The second portion of the duodenum is grasped and retracted medially so as to enable division of the peritoneum at its lateral border. An avascular plane is developed, with extension toward the midline to completely mobilize the duodenum through the third portion (FIG 4). The location of the ampulla is typically at the distal aspect of the second portion of the duodenum; hence, wide mobilization of the duodenum with a Kocher maneuver that extends inferiorly and medially toward the midline with full mobilization of the hepatic flexure to expose the third portion of the duodenum is essential to provide adequate exposure and a tension-free closure. This mobilization also allows the duodenum to be retracted up into the field where it may be maintained with stay sutures for subsequent steps of the operation. The placement of laparotomy pads posterior to the duodenum to further elevate it into the incision is recommended.
DUODENOTOMY AND EXPOSURE OF THE AMPULLA
■ Bimanually palpate the second portion of the duodenum to identify the lesion. If it cannot be palpated, place a biliary catheter via the cystic duct stump, inflate the balloon, and withdraw it until you meet resistance—the balloon will be palpable at the ampulla. An antimesenteric duodenotomy is then made to expose the luminal orifice of the ampulla (FIG 5
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








