Emmanuel A. Burdmann, Vivekanand Jha, Visith Sitprija
Acute Kidney Injury in the Tropics
Acute kidney injury (AKI) in the tropics is heavily influenced by the local disease burden, which is dominated by particular infections, consumption of unsafe drinking water, use of natural medicines, exposure to environmental toxins, and envenomation by snakes or arthropods. Poverty, malnutrition, lack of an adequate sanitary infrastructure and public health system, and uncontrolled and unplanned urbanization are frequent problems that add significantly to the observed disease burden. In fact, the rupture of the ecologic balance by humans has induced particular causes of renal injury in the tropics, such as accidents involving Africanized bees or Lonomia genus caterpillars.1,2
Acute kidney injury epidemiology in the tropical area has a peculiar, bimodal pattern. Most patients are encountered in the community, and previously healthy, young individuals are frequently affected. In contrast, the epidemiology of hospital-acquired AKI in large cities and tertiary hospitals is similar to that seen in nontropical countries.1–4
The uniqueness of the tropical environment has a direct influence on water quality and pattern of disease. A strong seasonal variation in the incidence of AKI is noted in the tropics, with a spike during and immediately after the rainy seasons. Heavy precipitation leads to soil erosion, leaching of minerals and organic compounds, and waterlogging, conditions favorable to survival of organisms causing or transmitting infectious diseases such as leptospirosis, dengue, and malaria.5–7 Tropical rains force venomous snakes to come out of the flooded burrows in the fields, making accidents more likely to occur.
The importance of the different causes of AKI in the tropics varies from region to region. Malaria, leptospirosis, typhus, diarrheal diseases, and envenomation are the common causes of AKI in South Asia; malaria, diarrheal diseases, obstetric accidents, and indigenous herbal remedies are frequent causes of AKI in Africa; and leptospirosis, dengue fever, envenomation, and obstetric complications are important causes of AKI in Latin American countries.8 In addition, classical AKI caused by rhabdomyolysis from heat stroke or overexertion may also occur (see Chapter 69).
Snakebites
Snakebite is an occupational hazard in the rural tropics and subtropical regions. Data based on 2005 Global Burden of Disease study suggest that globally at least 421,000 envenomations and 20,000 deaths occur each year as a result of snakebite, with the highest burden in South Asia, Southeast Asia, and sub-Saharan Africa.9 Renal injury may develop following bites by hemotoxic or myotoxic snakes belonging to the Viperidae and Elapidae families, such as the Russell viper, saw-scaled viper, puff adder, rattlesnake, tiger snake, and green pit viper, Bothrops species, Lachesis species, Crotalus species, the boomslang, gwardar, and dugite, Hypnale species, Cryptophis species, and sea snakes. AKI is more frequent following Russell viper, Bothrops, or Crotalus bites, with incidences ranging from 10% to 32% (Fig. 70-1).1,2,10–12 The prevalence is higher in children, probably because of the higher venom dose in relation to the body size.12
Clinical Features
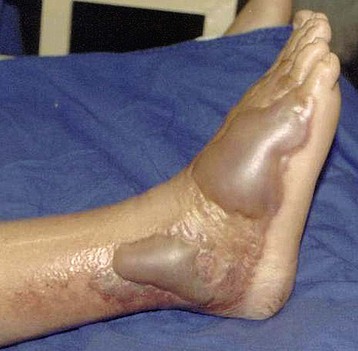
Manifestations depend on the nature and injected venom dose. Local pain, swelling, blistering, ecchymosis, and tissue necrosis are frequent in Russell viper and Bothrops bites (Figs. 70-2 and 70-3). The commonest systemic manifestation in accidents involving these snakes is coagulation abnormalities leading to bleeding diathesis (see Fig. 70-3). Muscle paralysis and rhabdomyolysis may occur following bites by sea snakes and Crotalus snakes. AKI develops within a few hours to as late as 96 hours after the bite. Cola-colored urine is noted in patients with hemolysis or rhabdomyolysis. AKI is usually oliguric and catabolic, with rapidly rising levels of blood urea nitrogen (BUN), serum creatinine, and potassium. Oliguria generally lasts for 1 to 2 weeks, and its persistence suggests the likelihood of acute cortical necrosis.1,2,10–12 Significant proteinuria and hematuria have been described in some studies and may reflect differences in venom composition.10,13
Laboratory investigation may show hemolysis (elevated free plasma hemoglobin and lactate dehydrogenase [LDH] and reduced haptoglobin) along with hypofibrinogenemia; reduced factors V, X, and XIIIa, protein C, and antithrombin C; and elevated fibrin degradation products. Rhabdomyolysis may be indicated by raised creatinine phosphokinase. Other findings include leukocytosis and elevated hematocrit resulting from hemoconcentration.1,2,10–12
Pathology
Grossly, the kidneys may show petechial hemorrhages. Light microscopy usually discloses acute tubular cell injury ranging from mild changes to overt tubular necrosis, with hyaline or pigment casts, variable degree of interstitial edema and infiltration, and scattered hemorrhages. Mesangiolysis may occur (especially with crotalid envenomation), and blood vessels may show fibrin thrombi. Electron microscopic findings include dense intracytoplasmic bodies representing degenerated organelles in the proximal tubules and electron-dense mesangial deposits. Less common findings are acute interstitial nephritis, necrotizing vasculitis, and proliferative and crescentic immune-complex glomerulonephritis (GN). Acute cortical necrosis is seen in about 20% to 25% of patients who have sustained Russell viper and Echis carinatus bites and has been described after Bothrops accidents.1,2,10–12
Pathogenesis
Snake venom is a complex mixture of enzymes, toxins, and peptides. Kidney injury may develop as a result of direct nephrotoxicity, renal vasoconstriction, hypovolemia, hypotension, depression of the medullary vasomotor center or the myocardium, hemolysis, altered fibrinolysis, myoglobinuria, or disseminated intravascular coagulation.1,2,10–12 Experimental studies have shown evidence of tubular injury manifested by increased excretion of tubular enzymes, altered fractional sodium excretion, and acute tubular necrosis.1,2,10–12,14 Other changes including mesangiolysis, disrupted integrity of the cellular junctions, proteolysis of extracellular matrix, lysis of vessel walls (leading to mesangiolysis), and alterations in function of enzymes vital to cellular integrity have been noted. 1,2,10–12 Recently, intense inflammatory mediator release, induction of oxidative stress, and release of mitochondrial alarmins have been evoked as possible pathogenic factors in snake venom–induced AKI.15–17
Management
The basic therapeutic approach is the same as that for AKI from any cause (see Chapters 73 and 74). Key steps for reducing morbidity and mortality include early administration of specific monovalent antivenom, appropriate volume replacement, maintenance of good urine flow, urinary alkalization in patients with rhabdomyolysis, correction of electrolyte imbalance, administration of tetanus immunoglobulin, and treatment of infections.1,2,10–12,14 Locally available polyvalent antivenom is effective against envenomation by multiple snakes or an unknown type of snake.18 Unavailability of antivenom in rural hospitals and poor infrastructure that hampers transportation to health centers are major factors that delay administration of antivenom and contribute to high mortality,19 which may reach 30%.1,2,10–12 Recent studies suggest that up to 40% of affected patients may be found to have developed chronic kidney disease (CKD) on long-term follow-up after snakebite-induced AKI.20,21
Arthropods
Poisonous arthropods such as bees, wasps, caterpillars, and spiders may cause AKI. Patients who have sustained hundreds of simultaneous bee stings frequently develop a multifaceted clinical picture, which includes intravascular hemolysis; rhabdomyolysis; low platelet count; coagulation disorders and bleeding; cardiovascular, hepatic, and pulmonary injury; and AKI, with mortality up to 16%.1,22,23 In the same way, AKI can follow incidents involving multiple wasp, yellow jacket, or hornet stings.22 The mechanisms leading to kidney injury are direct venom nephrotoxicity, intrarenal vasoconstriction, hemoglobinuria, myoglobinuria, hypotension, thrombotic microangiopathy,24 and activation of inflammatory and oxidative stress pathways.25 Renal histology usually shows acute tubular necrosis.1,22,24
Accidents involving caterpillars of the genus Lonomia produce severe hemorrhagic disorders. The venom induces a complex hemorrhagic diathesis with both fibrinolytic and disseminated intravascular coagulation–like activity.22,26 Severe and prolonged AKI with renal histology suggesting ischemic injury, which evolves to CKD in some patients, has been reported after Lonomia obliqua accidents (Fig. 70-4).22,27,28 Availability of Lonomia antivenom has led to an apparent decrease in the number of severe cases in Brazil.27 The pathogenesis of the kidney injury might be associated with glomerular deposition of fibrin microthrombi, intravascular hemolysis, kidney tissue deposition, and direct venom nephrotoxicity and activation of the endothelial cell inflammatory pathway.22,29–31
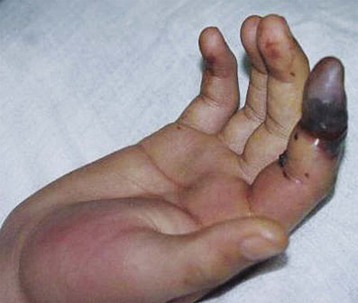
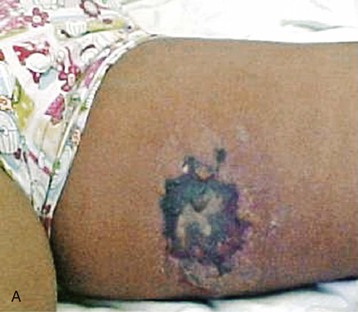
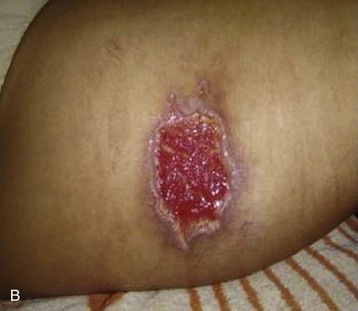
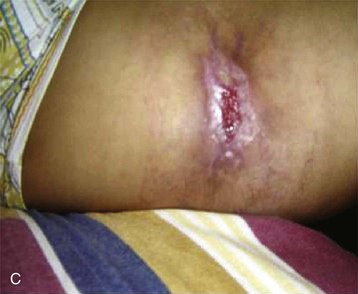
Spiders of the genus Loxosceles may induce local necrosis at the bite site, intravascular hemolysis, rhabdomyolysis, coagulation system changes, and AKI (Figs. 70-5 and 70-6).22,32 Even patients with mild cutaneous lesions may develop severe hemolysis and AKI, which is the main cause of death after these accidents. The pathogenesis of the kidney injury has been related to massive intravascular hemolysis, renal vasoconstriction, direct nephrotoxicity, and rhabdomyolysis.22,32–34

Natural Medicines
Herbs and indigenous remedies are used extensively in poor societies living in the tropics. About 80% of the population in Africa depends on traditional medicine prepared by herbalists and faith healers using unknown ingredients. Poisoning with traditional medicines is an important cause of renal injury and mortality in sub-Saharan Africa.35–37 AKI has been described in association with several natural remedies. An accurate assessment of their contribution to AKI is rendered difficult by the failure to elicit a history as a result of the physicians’ ignorance or denial or the patients’ fear of stigmatization or social pressure. Often it is difficult to discount the contribution of the original illness to the AKI.8,35–37
About 25% to 35% of all AKI from medical causes in African hospitals are related to herbal remedies. The plant sources more frequently associated with AKI are impila (Callilepis laureola), djenkol beans (Pithecellobium), irumban puli (Averrhoa bilimbi), mushrooms (genera Amanita, Galerina, Cortinarius, and Inocybe), cape aloe, and raw carp bile.8,36,37 In addition, single case reports of other natural medicines inducing AKI have been described (Table 70-1).8,36,37
Table 70-1
Herbal causes of acute kidney injury (AKI) in tropical countries.
DIC, Disseminated intravascular coagulation.
| Herbal Causes of Acute Kidney Injury in Tropical Countries | ||||
| Plant (Common or Local Name) | Country | Active Molecule | Renal Manifestations | Other Manifestations |
| Averrhoa bilimbi (irumban puli) | South India | Oxalic acid | Intratubular obstruction | |
| Callilepis laureola (impila) | Sub-Saharan Africa | Atractyloside | Acute tubular necrosis | Abdominal pain, diarrhea, vomiting, jaundice, seizures, and coma |
| Catha edulis (khat leaf) | East Africa, Arabian peninsula | S-cathinone and ephedrine | Acute tubular necrosis | Hepatotoxic effects |
| Cleistanthus collinus (oduvan) | India | Cleistanthin A and B, collinusin, and diphyllin | AKI | Hypotension, hypokalemia, arrhythmia |
| Colchicum autumnale (autumn crocus) | Turkey | Colchicine | Acute tubular necrosis | Hemorrhagic gastroenteritis, muscle paralysis, respiratory failure |
| Crotalaria laburnifolia (bird flower) | Zimbabwe, Sri Lanka | Pyrrolizidine alkaloids | Acute tubular necrosis and hepatorenal syndrome | Hepatic veno-occlusive disease, pulmonary injury, thrombocytopenia |
| Dioscorea quartiniana (yam) | Africa, Asia | Dioscorine and dioscin | Acute tubular necrosis | Convulsions |
| Pithecellobium lobatum and Pithecellobium jiringa (djenkol beans) | South East Asia | Djenkolic acid | Intratubular obstruction and acute tubular necrosis | Lumbar and lower abdominal pain, hypertension |
| Dodonaea angustifolia (sand olive) | South Africa | Unknown | Acute interstitial nephritis | Pulmonary embolism |
| Euphorbia metabelensis (spurge) | Zimbabwe | Irritant chemicals in plant latex | Acute tubular necrosis | Thrombocytopenia |
| Larrea tridentate (chaparral) | Chile, South Africa | Nordihydroguaiaretic acid and S-quinone | Renal cysts, renal cell carcinoma | Hepatic failure |
| Propolis resin | Brazil | |||