Kevan R. Polkinghorne, Peter G. Kerr
Acute Complications During Hemodialysis
Although technical advances in hemodialysis (HD) have made the procedure increasingly safe and well tolerated, there are still important acute complications that will be encountered by physicians responsible for patients receiving HD in both acute and chronic clinical settings. These complications with their causes and management are discussed in this chapter.
Cardiovascular Complications
Intradialytic Hypotension
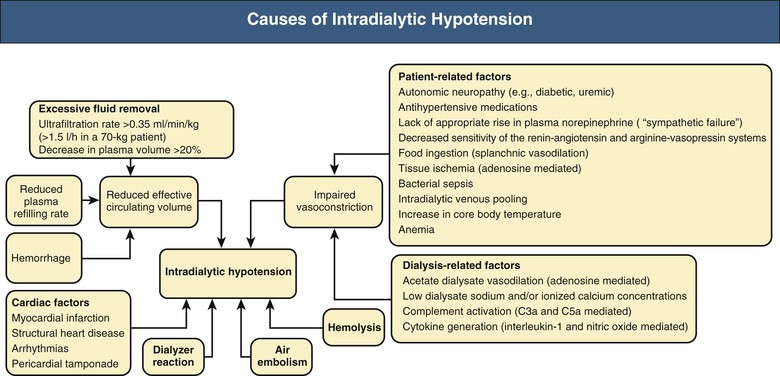
Intradialytic hypotension, which occurs in 10% to 30% of treatments, ranges from asymptomatic episodes to marked compromise of organ perfusion resulting in myocardial ischemia, cardiac arrhythmias, vascular thrombosis, loss of consciousness, seizures, or death.1 Furthermore, in patients with acute kidney injury, intradialytic hypotension may induce more renal ischemia and retard recovery of renal function. Intradialytic hypotension and postdialysis orthostatic hypotension have been shown to be independent risk factors for mortality.2 The pathogenesis of intradialytic hypotension is complex3 and is summarized in Figure 95-1. Most commonly, however, intradialytic hypotension results from the need to deal with excessive fluid weight gain between dialysis treatments. The subsequent rate of fluid removal required exceeds the achievable rate of intravascular filling, resulting in relative intravascular volume depletion.
The immediate treatment is to restore the circulating blood volume by placing the patient in the Trendelenburg position, reducing or stopping ultrafiltration, and infusing boluses of 0.9% isotonic saline (100 ml or more, as necessary). Salt-poor albumin and other hypertonic solutions offer no advantage over isotonic saline and cost more. Blood flow rate should not be routinely reduced to manage hypotension, because this has not been shown to be beneficial. If the blood pump rate is reduced transiently, particular attention should be paid to minimizing underdialysis from such a practice. Of course, in the setting of excessive weight gain, the administration of boluses of saline makes achieving the target dry weight more difficult. Because cardiac factors can precipitate intradialytic hypotension, the clinician should maintain a high index of suspicion for cardiac ischemia, especially if hypotension is accompanied by chest pain or dyspnea, and an electrocardiogram and serum troponins should be obtained. Similarly, recurrent and unexplained episodes of hypotension might warrant echocardiography to rule out pericarditis or pericardial effusion.
Preventive strategies include correction of anemia and hypoalbuminemia and treatment of congestive heart failure or arrhythmias, avoidance of antihypertensive drugs before dialysis, and avoidance of food before and during dialysis. Patients should be counseled to avoid excessive interdialytic weight gain, and accurate assessment of the patient’s dry weight is required. Particular attention should be given to ensuring minimal salt intake. Midodrine, an oral selective α1-agonist, 5 to 10 mg predialysis, is a useful preventive therapy.4
Preventive strategies through modification of the dialysis procedure include in the first instance use of bicarbonate dialysate, volumetric control of ultrafiltration, and sodium modeling. Subsequently, reducing the ultrafiltration rate by increasing either the number of hours or the frequency of dialysis can be tried.5 I On-line blood volume monitoring and biofeedback techniques have been developed in an attempt to improve intradialytic cardiovascular stability.6 Although on-line blood volume devices have been shown to decrease the incidence of intradialytic hypotension in an at-risk population, there is limited evidence that blood volume monitoring can predict intradialytic hypotension in individual patients or produce a long-term morbidity and mortality benefit, especially in the wider HD population.7 Cooling of dialysate to 35.5° C to 36° C (95.9° F to 96.8° F), a measure that induces release of catecholamines, resulting in vasoconstriction or at least preventing vasodilation, may lessen hypotension. A systematic review of the randomized controlled trials using biofeedback modulating dialysate temperature demonstrated significant reductions in the incidence of intradialytic hypotension compared with conventional dialysis.8 Modulation of dialysate temperature is achieved by measuring the blood temperature in the arterial and venous circuits and feeding back the information to the arterial and venous thermostats in the dialysis machine. The machine can be programmed to allow either a constant body temperature and a negative overall energy transfer, so-called isothermic hemodialysis, or thermoneutral hemodialysis, which aims to prevent energy transfer between the dialysate and extracorporeal blood. Both isothermic and thermoneutral HD have been shown to reduce intradialytic hypotension in randomized trials compared with conventional HD.
Intradialytic Hypertension
Intradialytic hypertension occurs in 8% to 30% of treatments.9 Hypertension during or immediately after HD constitutes an important risk factor for cardiovascular mortality. Moreover, an intradialytic increase in systolic blood pressure is associated with an increased risk of hospitalization or death.10
In most circumstances, an intradialytic elevation of blood pressure indicates significant volume overload. However, in a number of patients, blood pressure remains elevated despite fluid removal, a syndrome called dialysis-refractory hypertension. These patients are usually young with preexisting hypertension and have excessive interdialytic weight gain and a hyperactive renin-angiotensin system in response to fluid removal.11 In these patients the hypertension is still mediated by the previous volume expansion, but there is often a lag of days to 2 or more weeks before the blood pressure becomes normal (known as the dialysis “lag phenomenon”). Often patients can discontinue most of their antihypertensive agents once this occurs.
Erythropoietin (EPO) and other erythropoiesis-stimulating agents have been associated with a 20% to 30% incidence of new onset or exacerbation of hypertension. Furthermore, among patients receiving intravenous (not subcutaneous) EPO, elevated levels of endothelin-1 (a potent vasoconstrictor) have been shown to correlate with increased blood pressure. Predominantly, such EPO-induced rises in blood pressure are associated with a rapid rise in hemoglobin and can be avoided by a more conservative approach to correcting the hemoglobin.
Intradialytic hypertension can be precipitated by the use of high sodium dialysate, which is intended to mitigate the intradialytic decrease in serum osmolality that occurs with the diffusive removal of urea and sodium.12 Although this approach stabilizes blood pressure during dialysis by improving intravascular filling, high sodium dialysate results in a positive intradialytic sodium balance and is associated with increased postdialysis thirst, resulting in significant weight gain in the interdialytic period. To circumvent these problems, sodium modeling has been adopted as an approach that uses variable sodium concentrations in the dialysate, generally with sodium reduced in a continuous or stepwise manner from an initial level of 150 to 154 mmol/l to 138 to 142 mmol/l. Although sodium modeling has been widely promoted, randomized trial evidence for definite benefits of this approach is lacking.13 Other hypothesized mechanisms of intradialytic hypertension include hyperactivity of the sympathetic nervous system14 and increased cardiac output resulting from fluid removal, particularly among patients with cardiomyopathy.15 Clinicians should also be aware of possible dialytic removal of certain antihypertensive drugs, such as angiotensin-converting enzyme (ACE) inhibitors and beta blockers.
Increasing hypertension during a dialysis session requires intervention if systolic blood pressure is greater than 180 mm Hg. This is best treated with a centrally acting agent such as clonidine or a short-acting ACE inhibitor such as captopril. Successful treatment of hypertension for a longer period requires an accurate determination of the patient’s dry weight and its achievement by gradual ultrafiltration during several weeks of dialysis. Once dry weight is achieved, optimization of antihypertensive drug therapy is warranted, including the use of minimally dialyzable or nondialyzable medications such as angiotensin receptor blockers, calcium channel blockers, clonidine, and carvedilol. However, it should be remembered that use of antihypertensive agents may make achievement of dry weight difficult because of induced intradialytic hypotension. Evidence from the Dry-Weight Reduction in Hypertensive Hemodialysis Patients (DRIP) trial16 would suggest that optimal control of blood pressure in HD patients is via volume control, not the use of antihypertensive agents. One common error in management is to treat dialysis hypertension with increasing blood pressure medications as opposed to opting to achieve dry weight. The use of vasodilator drugs (hydralazine, minoxidil) can lead to increased fluid retention that worsens hypertension and volume overload. Minoxidil can also be associated with the development of pleural and pericardial effusions.
Cardiac Arrhythmias
Intradialytic arrhythmias are common and are often multifactorial in origin.17,18 Left ventricular hypertrophy, congestive cardiomyopathy, uremic pericarditis, silent myocardial ischemia, and conduction system calcification are frequently encountered in adult dialysis patients. In addition, polypharmacy coupled with the constant alterations in fluid, electrolyte, and acid-base homeostasis may precipitate intradialytic arrhythmias. The range of electrocardiographic abnormalities that may be encountered in renal failure is shown in Table 95-1. QTc dispersion, the difference between maximum and minimum QTc interval on a standard 12-lead electrocardiogram, is prolonged after HD and has been proposed as a prognostic indicator of cardiac complications in dialysis patients.19
Table 95-1
Electrocardiographic abnormalities in renal failure.
Risk factors include LV dysfunction, wall motion abnormalities, known coronary artery disease, abnormal perfusion scans (even without coronary artery disease), use of cardiac glycosides, and low dialysate potassium concentration.
| Electrocardiographic Abnormalities in Renal Failure | |
| Function | Abnormality Seen in Renal Failure |
| PR interval | Usually normal; prolongation in long-term hemodialysis Calcification of mitral valve annulus may involve His bundle, resulting in complete heart block |
| QRS interval | |
| Amplitude | Increases during ultrafiltration (correlates with reduction in left ventricular [LV] dimensions) LV hypertrophy (LVH) on voltage criteria found in up to 50% |
| Duration | Prolonged (within normal range) by hemodialysis Late potentials increased only in patients with preexisting coronary heart disease Prolonged in hyperkalemia |
| ST segment | Depression during hemodialysis does not predict coronary artery disease Depression or elevation may occur in hyperkalemia Depression during ambulatory monitoring poorly predictive of coronary artery disease |
| QTc interval | Increases during hemodialysis (correlates with reduction in K+ and Mg2+) Increased QT dispersion reported in patients on dialysis |
| T wave | Peaking or inversion may occur in hyperkalemia Inversion in anterolateral leads in LVH with strain pattern |
| Rhythm | High incidence of atrial and ventricular arrhythmias during hemodialysis |
Preventive measures include the use of bicarbonate dialysate and careful attention to dialysate potassium and calcium levels. There are very few trials of the most appropriate dialysate potassium level, although recent observational evidence suggests that dialysate potassium levels below 2 mmol/l should be avoided in most patients.20 Zero potassium dialysate should be avoided because of its arrhythmogenic potential, particularly in patients receiving digoxin, should serum potassium concentration decrease to less than 3.5 mmol/l. Serum digoxin levels should be regularly monitored and the need for the drug regularly reassessed.
Sudden Death
Cardiac arrest occurs at a rate of 7 per 100,000 HD sessions and is more common in the elderly, patients with diabetes, and patients using central venous catheters.21 Some 80% of sudden deaths during dialysis are caused by ventricular fibrillation, more frequently observed after the long interdialytic interval on thrice-weekly dialysis, presumably because of more marked fluid and solute accumulation.22–24 Consistent with this hypothesis, both peritoneal dialysis patients and patients undergoing long-duration HD do not show this high event rate on a particular day of the week.24 Although coronary heart disease increases the risk of sudden death, other catastrophic intradialytic events need to be ruled out. The prompt recognition and treatment of hyperkalemia, often encountered in young, noncompliant patients, is imperative. Profound generalized muscle weakness may be a warning sign of imminent life-threatening hyperkalemia.
When cardiopulmonary arrest occurs during dialysis, an immediate decision must be made as to whether the collapse is the result of an intrinsic disease or technical errors, such as air embolism, unsafe dialysate composition, overheated dialysate, line disconnection, or sterilant in the dialyzer. Air in the dialysate, grossly hemolyzed blood, and hemorrhage caused by line disconnection can be easily detected. However, if no obvious cause is identifiable, blood should not be returned to the patient, particularly if the arrest occurred immediately on initiation of dialysis. A patient exposed to formaldehyde may have complained earlier of burning at the access site; fortunately this agent is rarely used today. If the possibility of a problem with dialysate composition is remote, blood may be returned to the patient. However, blood and dialysate samples should be immediately sent for electrolyte analysis, the dialyzer and blood lines saved for later analysis, and the dialysis machine replaced until all its safety features have been thoroughly evaluated for possible malfunction. It should be a standard practice to have defibrillators in dialysis units. The management of cardiopulmonary arrest during dialysis should follow the standard principles of cardiopulmonary resuscitation; the diagnosis and management of technical errors are discussed later.
Prevention of sudden cardiac death in HD patients, including the role of implantable defibrillators, is further discussed in Chapter 82.
Dialysis-Associated Steal Syndrome
The construction of an arteriovenous fistula or graft frequently results in reduction of blood flow to the hand. Although clinically significant ischemia does not usually result, symptoms are by no means rare, particularly in diabetic or elderly patients with peripheral vascular disease. Dialysis-associated steal syndrome is more common in upper arm arteriovenous fistulas (about 4%) compared with both arteriovenous grafts and forearm arteriovenous fistulas (about 1%). The clinical presentation, differential diagnosis, and evaluation of dialysis-associated steal syndrome are summarized in Box 95-1 and are discussed further in Chapter 91.25,26
Treatment depends on the clinical severity of ischemia and vascular access anatomy.26 Severe ischemia can cause irreparable injury to nerves within hours and must be considered a surgical emergency. Mild ischemia, manifested by mild pain during HD, subjective coldness and paresthesias, and objective reduction in skin temperature but with no loss of sensation or motion, is common and generally improves with time.27 Patients with mild ischemia should undergo symptom-specific therapy (e.g., wearing a glove) and frequent physical examination, with special attention to subtle neurologic changes and muscle wasting.28 Failure to improve may necessitate surgical or radiologic intervention (see Chapter 91). Persistence of symptoms after an apparently successful correction of the vascular access flow should alert the clinician to other unrelated causes.
Neuromuscular Complications
Muscle Cramps
Muscle cramps occur in 5% to 20% of patients late during dialysis and frequently involve the legs. They account for 15% of premature discontinuations of dialysis.30 Electromyography shows increased tonic muscle electrical activity throughout dialysis, and serum creatine kinase may be elevated.
Although the pathogenesis is unknown, dialysis-induced volume contraction and hypo-osmolality are common predisposing factors. Indeed, the onset of muscle cramps may give an indication that the target weight has been reached. However, hypomagnesemia and carnitine deficiency may also play a role.
The acute management is directed at increasing plasma osmolality. Cessation of ultrafiltration is not useful. Parenteral infusion of 23.5% hypertonic saline (15 to 20 ml), 25% mannitol (50 to 100 ml), or 50% dextrose in water (25 to 50 ml) is equally effective. However, hypertonic saline may result in postdialytic thirst, and both hypertonic saline and mannitol cause transient warmth and flushing during the infusion. Furthermore, large and repetitive infusions of mannitol may lead to increased thirst, interdialytic weight gain, and fluid overload. Overall, dextrose in water is preferred, particularly in nondiabetics.
Preventive measures include dietary counseling about excessive interdialytic weight gain. In patients without clinical signs of fluid overload, it is reasonable to increase the dry weight by 0.5 kg and to observe the clinical response. Quinine sulfate (250 to 300 mg) or oxazepam (5 to 10 mg) given 2 hours before dialysis may also be effective. Although the U.S. Food and Drug Administration regards quinine sulfate as both unsafe and ineffective for the prevention of cramps, this drug works well in some patients, and it is used freely in most parts of the world. Some reports also promote the use of vitamin E in this role.31 The use of sodium gradient during dialysis may have some benefit as well. Proposed strategies include starting with a dialysate sodium concentration of 145 to 155 mmol/l and a linear decrease to 135 to 140 mmol/l by the completion of the treatment. A comparison of sodium modeling with an exponential, linear, or step program has yielded similar results.32 In anecdotal reports, 5 mg of enalapril twice weekly may be effective, presumably by inhibiting angiotensin II–mediated thirst. Stretching exercises, magnesium, creatine monohydrate (12 mg before dialysis),33 and l-carnitine supplementation (20 mg/kg per dialysis session) may also be beneficial.34 An intradialytic blood volume biofeedback control system has been shown to reduce the incidence of muscle cramps.35
Restless Legs Syndrome
Restless legs syndrome is common in dialysis patients. The typical complaint is of crawling sensations in the legs that occur with inactivity, and symptoms may worsen during dialysis. The etiology, prevention, and management of restless legs syndrome are discussed in Chapter 86.
Dialysis Disequilibrium Syndrome
Despite a decline in its incidence, dialysis disequilibrium syndrome (DDS) is still observed sporadically in patients who are initiated on HD on high-flux dialyzers with large surface areas and short dialysis time. Risk factors include young age, severe uremia, rapid and marked intradialytic falls in urea at dialysis initiation, low dialysate sodium concentration, and preexisting neurologic disorders (see Chapter 86).
Dialysis disequilibrium syndrome commonly presents with restlessness, headache, nausea, vomiting, blurred vision, muscle twitching, disorientation, tremor, and hypertension. More severe manifestations include obtundation, seizures, and coma. DDS usually develops toward the end of dialysis but may be delayed for up to 24 hours. Although cerebral edema is a consistent finding on computed tomographic scanning, DDS remains a clinical diagnosis because laboratory tests, including electroencephalography, are nonspecific. It is usually self-limited, but full recovery may take several days.
The pathogenesis of DDS is still a subject of debate. The reverse urea effect theory, which proposes that a transient osmotic disequilibrium occurs during dialysis as a result of a more rapid removal of urea from blood than from cerebrospinal fluid, has been disputed.36 In animals undergoing rapid dialysis, despite the correction of systemic acidosis, a paradoxical cerebrospinal fluid acidosis develops that is aborted by slower dialysis. An additional mechanism is the intracerebral accumulation of idiogenic osmoles, such as inositol, glutamine, and glutamate.
In high-risk patients, preventive measures include the use of volumetric-controlled machines, bicarbonate dialysate, sodium modeling, earlier recognition of uremic states, and stepped initiation of dialysis (short initial treatment times with lower blood pump speeds). In addition, short and more frequent dialysis treatments are recommended with use of small surface area dialyzers and reduced blood flow rates. The target reduction in blood urea should initially be limited to 30%. The prophylactic use of mannitol or anticonvulsants is not recommended.
An extension of this syndrome may be one that mimics osmotic demyelination syndrome—similar to that seen with rapid correction of hyponatremia. Several cases have been reported in association with dialysis initiation, with clinical manifestations similar to the locked-in pontine picture of central demyelination. The difference is that with the dialysis-related condition, the patients appear to recover over the ensuing 5 to 7 days and the condition seems to be related to edema rather than demyelination.37
Seizures
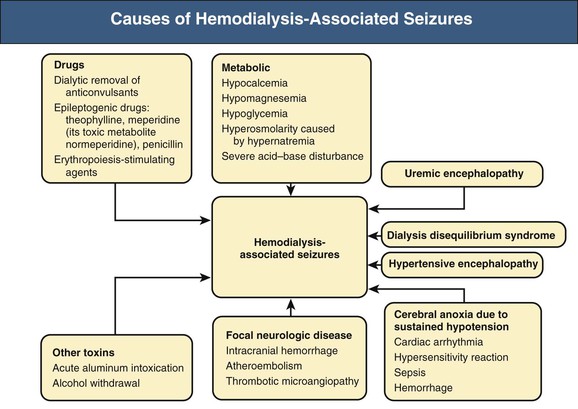
Intradialytic seizures occur in less than 10% of patients and tend to be generalized but easily controlled. However, focal or refractory seizures warrant evaluation for focal neurologic disease, particularly intracranial hemorrhage. Causes of seizures are summarized in Figure 95-2 and are discussed further in Chapter 86.
Treatment of established seizures requires cessation of dialysis, maintenance of airway patency, and investigation for metabolic abnormalities. Intravenous diazepam, alprazolam or clonazepam, and phenytoin may be required. Intravenous 50% dextrose in water should be administered promptly if hypoglycemia is suspected.
Headache
Dialysis headache is common and consists of a bifrontal discomfort that develops during dialysis and may become intense and throbbing, accompanied by nausea and vomiting. It is usually aggravated by the supine position, but there are no visual disturbances.
Although its cause has not been elucidated, dialysis headache may be a subtle manifestation of DDS or may be related to the use of acetate, which is present in low concentrations (3 to 4 mmol/l) in almost all dialysate fluid. A role for nitric oxide has also been postulated.38 Alternatively, it may be a manifestation of caffeine withdrawal caused by dialytic removal of caffeine.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree