Chapter 18 Achalasia
Etiology
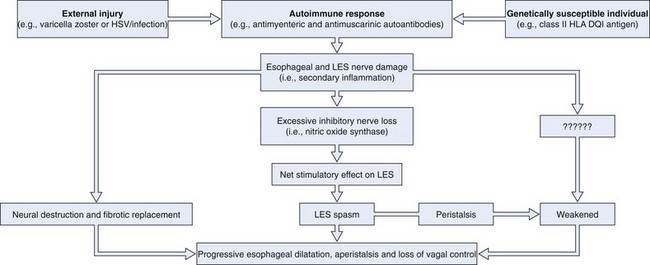
Fig. 18.1 illustrates the putative events in the development of achalasia. Extensive experimental data support this hypothesis. First, the neurotropic virus, varicella zoster, has been detected by in situ hybridization in cardiomyotomy specimens from patients with achalasia.1 More recent data have also implicated herpes simplex virus type 1 as a cause of achalasia by showing a population of cells and cytokines in LES tissue typically found in herpes simplex virus infection.2 In addition, Chagas’ disease, a disease caused by Trypanosoma cruzi, a protozoan that is endemic in Central and South America, causes esophageal disease that may be indistinguishable from achalasia on manometric and radiographic grounds as a consequence of denervation of Auerbach’s plexus.3 Second, some investigators have shown an association between white patients with achalasia and the class II human leukocyte antigen (HLA) DQ1.4 This genetic relationship has been characterized more specifically as an association with the DQB1*0602 and DRB1*15 HLA alleles.5 Third, antimyenteric6 and antimuscarinic cholinergic receptor autoantibodies7 have been detected in patients with achalasia. Fourth, studies of surgical specimens from patients with achalasia have shown a chronic inflammatory infiltrate leading to ganglionic destruction and fibrosis.8–12 Fifth, vagal dysfunction has been shown in natural and experimental animal models of achalasia.13,14 Sixth, a selective deficiency of nitric oxide synthase, the predominant inhibitory neurotransmitter of the LES, has been shown in animal models and patients with achalasia.15,16 Seventh, patients with less advanced forms of achalasia (from both a clinical and a radiographic point of view) have more inflammation and less fibrosis in the ganglia of the LES than patients with more advanced forms of the disease,11,17 suggesting that LES basal and residual tone may increase and esophageal body function deteriorates as the disease progresses.18
Much more data are needed to prove this hypothesis. The data referred to were generated from a relatively small number of patients, given the rarity of the disease. The abnormalities described are rarely identified in all patients with achalasia. Only 3 of 9 patients had detectable varicella zoster in the myenteric plexus,1 and only 7 of 18 patients had antimyenteric neuronal antibodies.5 These preliminary findings are encouraging, however, particularly because the general theory proposed here may apply to other gastrointestinal (GI) disease states in addition to achalasia (e.g., chronic idiopathic pseudoobstruction).
Diagnosis
Symptoms
The hallmark symptoms of achalasia are dysphagia, chest pain, and regurgitation (Table 18.1). Various series of patients with achalasia have reported dysphagia in 82% to 100% of cases, chest pain in 17% to 95%, and regurgitation in 59% to 81%.19–24 Dysphagia occurs typically for both solids and liquids, but solid food dysphagia often precedes liquid dysphagia. Some patients with early disease describe the need to “wash down their food with water,” which possibly affords relief by raising a sufficient head of pressure above to overcome the LES spasm below. In others, there may be superimposed acute episodes of solid food dysphagia in which patients present with symptoms suggestive of food impaction that resolve spontaneously or are severe enough to warrant endoscopic removal.
Table 18.1 Symptoms of Achalasia
| Symptoms | Frequency (%)* |
|---|---|
| MAJOR | |
| Dysphagia | 82–100 |
| Chest pain | 17–95 |
| Regurgitation | 59–81 |
| Weight loss | 32 |
| MINOR | |
| Slow eating | |
| Stereotypic maneuvers during eating | |
| Halitosis | |
| Heartburn | |
| Accumulation of oral debris at night | |
| Staining of pillow during sleep | |
| Nocturnal coughing or choking | |
| Acute airway obstruction | |
| Inability to belch | |
| Postprandial syncope | |
| Dental caries | |
| Asthma | |
| Pneumonia |
Chest pain often occurs during ingestion and may signify food impaction. A spasmlike chest pain that may mimic gastroesophageal reflux disease (GERD) (i.e., heartburn) or cardiac pain (i.e., angina) may also occur spontaneously. The origin of the chest pain is generally unclear, and it may be independent of the mechanisms that cause dysphagia and regurgitation; this is suggested by data showing that chest pain commonly persists after achalasia treatment that is effective for the other two major symptoms.24
Regurgitation may occur minutes to hours after ingestion of a meal. Patients may even identify food that they ingested many days before in the regurgitant. Patients with severe symptoms may be unable to drink a glass of water without regurgitating. The nature of the regurgitant may be useful to differentiate achalasia from GERD. In achalasia, the regurgitant is typically described as nonsour or nonbitter, and patients may mention that it “tastes just like it did when it was initially swallowed.” Because of the difficulty with eating, weight loss is also a common feature of achalasia; this was seen in 32% of patients in one more recent study.25
In our experience, a careful history emphasizing and dissecting the major symptoms of dysphagia, chest pain, regurgitation, and weight loss can be quite specific for the diagnosis of achalasia, although we are constantly surprised by the varied patterns of presentation of this unusual disease. As with many chronic disease states in which life-threatening consequences are rare and take months to years to develop, achalasia is a disease that lends itself to self-accommodation on the part of the patient. Behavioral adaptation may lead to the emergence of a whole host of subtle compensatory symptoms accompanied by the patient denying or downplaying the more classic symptoms described previously (see Table 18.1).
One more recent study25 elicited numerous compensatory symptoms, including slow eating and other stereotypic maneuvers to aid swallowing such as walking, standing, sitting straight up, or arching of the neck during swallowing. Potential patients should also be interviewed about other subtle symptoms, including halitosis, heartburn, accumulation of oral debris or staining of the pillow during sleep, nocturnal coughing or choking, acute airway obstruction,26,27 an inability to belch because of upper esophageal sphincter dysfunction,28,29 and postprandial syncope.30 Heartburn, the cardinal symptom of gastroesophageal reflux, is particularly important because its presence is often erroneously believed to be a strong negative indicator of achalasia. Although unproved, the sensation may be a consequence of bacterial fermentation of retained ingested foodstuff leading to lowering of the esophageal luminal pH mimicking GERD. Some studies have shown that heartburn and specialist referral of patients on proton pump inhibitors can be quite common in the early stages of achalasia.25,31 Other important symptoms often erroneously ascribed to GERD include the presence of dental caries (from food bathing the teeth at night), asthma, and a history of pneumonia (presumably from microaspiration). Accurately differentiating between GERD and achalasia by history alone may be surprisingly difficult, given that the two diseases represent completely opposite ends of the pathophysiologic spectrum.
Radiography
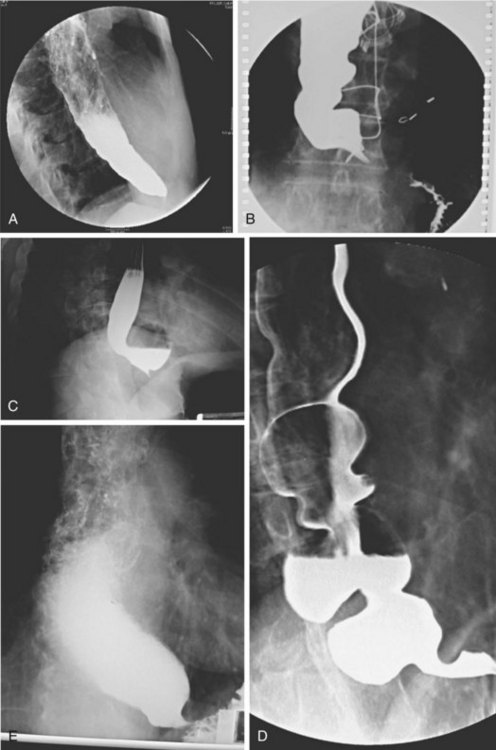
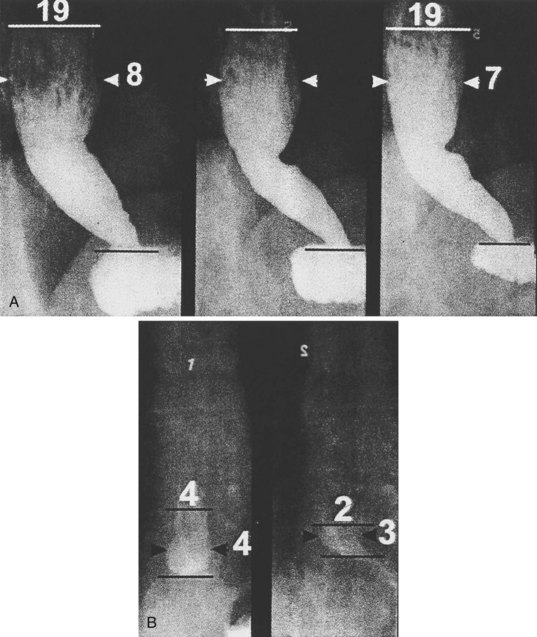
Achalasia may be suspected on the basis of a routine chest x-ray film (particularly a lateral view) by the presence of an air-fluid level or a dilated esophagus or both. However, barium swallow is the most reliable radiographic method for making the diagnosis (Fig. 18.2). The pathognomonic findings include a smooth, tapered narrowing of the gastroesophageal junction (the so-called bird’s beak appearance) with a proximally dilated esophagus that may be filled with fluid or food debris. Features of candidal infection, a consequence of long-standing esophageal stasis, may also be present. However, this basic template can include remarkable heterogeneity. The degree of esophageal dilation may vary from minimal to massive, and end-stage disease may reveal sigmoidization of the esophagus. Similarly, the diameter of the gastroesophageal junction may range from less than 1 mm to greater than 8 mm. Radiographs may also show single or multiple distal esophageal diverticula of varying sizes or a corkscrew or “spastic” appearance with several simultaneous lumen-obliterating contractions (this has been termed vigorous achalasia).
To assess and characterize these esophageal radiographic findings, various scoring systems have been devised.19,25 Although these scoring systems help to standardize interpretation of radiographic appearance, which may be useful from a research point of view, there is generally very poor correlation between symptom severity and radiographic score.25 This limitation is discussed further in the treatment section. Finally, there has been an old tenet in esophagology that a hiatal hernia is unusual in achalasia.32 More recent data have questioned this dictum, showing the presence of a hiatal hernia in 10 of 71 radiographs reviewed from patients with achalasia (14.5%) compared with 9 of 35 patients (25.7%) 51 years old or older, a frequency that is similar to the normal population.33 This finding makes intuitive sense because achalasia may develop at any age, sometimes in the presence of long-standing GERD with a hiatal hernia that preceded the development of the achalasia. Perhaps older studies had a bias of either underdiagnosis of hiatal hernia or overenrollment of younger patients.
Esophageal Manometry
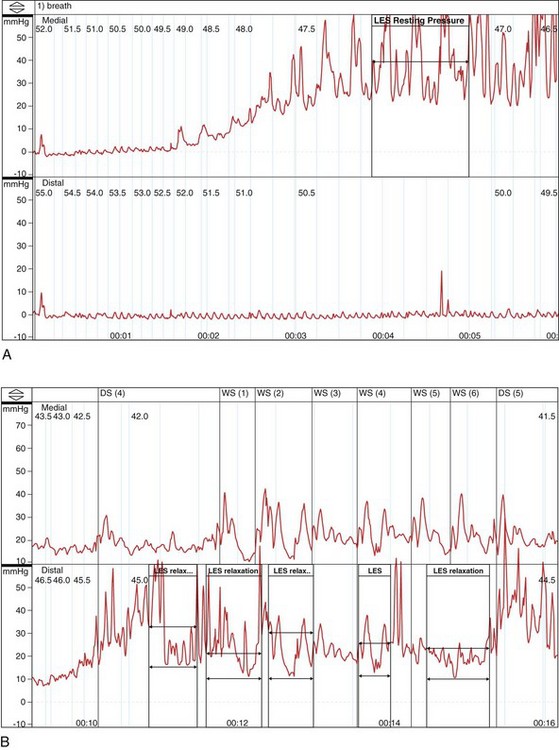
In the absence of a true “gold standard,” manometry is the best test for the diagnosis of achalasia (Fig. 18.3). However, manometric diagnosis of achalasia has undergone great revision in recent years. Classic teaching required the presence of a hypertensive LES with incomplete relaxation (i.e., an increased residual pressure) and esophageal body aperistalsis, whereas it is now clear that these classic criteria do not occur in all patients. Part of the reason for this reevaluation has been prolonged combined ambulatory pH and manometric recordings in which peristaltic contractions, complete LES relaxation, and acid reflux may be shown intermittently in patients who otherwise fulfill the classic criteria on stationary manometry.34 As a result, it now seems conceivable that “normal” manometric findings might occur on stationary manometry as well in patients with achalasia, depending on the timing of the study.
Hirano and coworkers35 in a study of 58 patients with achalasia proposed a manometric heterogeneity in patients with achalasia, including features such as a normal basal LES pressure, normal residual pressures, high-amplitude contractions, varying lengths of aperistalsis, and the presence of transient LES relaxations (the hallmark of GERD). In interpreting this important study, one might wonder what the “gold standard” for the diagnosis of achalasia was in these “atypical” patients. This study shows the importance of making the diagnosis of achalasia based on a combination of compatible clinical, radiographic, and manometric findings in a specific patient rather than routinely relying on classic criteria. Similarly, Vantrappen and coworkers36 in a landmark article proposed that diffuse esophageal spasm is in itself one end of the spectrum of achalasia. Several case reports have now shown spasm-type manometric (and perhaps radiographic) findings either as a component of established achalasia or as an early manifestation of dysmotility that may evolve into achalasia in due course.37,38
Currently, many esophagologists consider diffuse esophageal spasm a true variant of achalasia. Attention has also focused on the concept of vigorous achalasia, which is distinguished from classic achalasia by the presence of high-amplitude nonperistaltic esophageal body contractions. Vigorous achalasia may also represent an early manometric manifestation of the disease, before the development of complete aperistalsis. Although pathologic studies have shown progression of histologic injury within the myenteric plexus during the transition from vigorous to classic achalasia,10 clinical studies comparing radiographic appearance and response to therapy in vigorous and classic achalasia have not borne out these differences.39
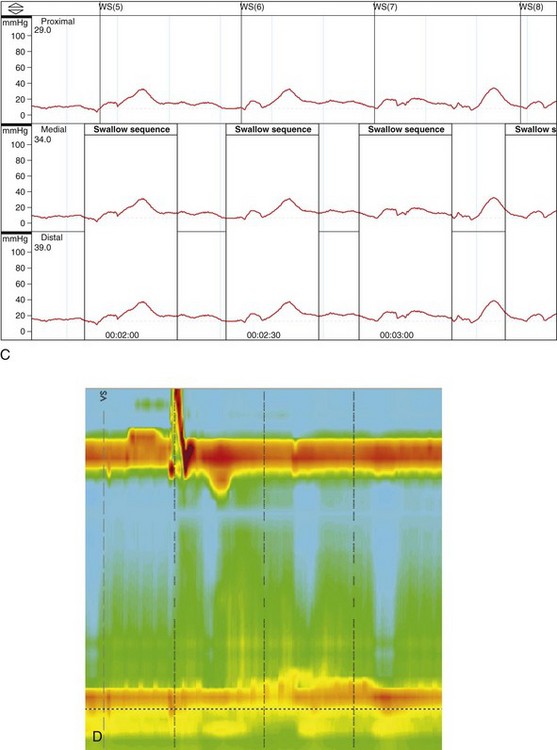
Significant advances have been made in motility testing of the esophagus in recent years with the advent of high-resolution manometry.40–42 This technique expands on prior techniques by permitting simultaneous manometry measurements at centimeter intervals along the entire length of the esophagus. Three-dimensional color plots of change in pressure measurements with time (pressure topography) allow more accurate recording of the upper esophageal sphincter and LES pressures and the proximal (striated) and distal (smooth) muscular contractions of the esophageal body. These sophisticated tracings have permitted a new manometric classification of achalasia as proposed by Kahrilas and colleagues.40 This new classification divides achalasia into three manometric types: type I, achalasia with minimal esophageal pressurization, previously referred to as classic achalasia (with aperistalsis and an elevated LES pressure that fails to react; see Fig. 18.3D), occurring in roughly 21% of cases; type II, achalasia with esophageal compression, previously referred to as vigorous achalasia (with swallow-induced pressurization), occurring in roughly 49% of cases; and type III, achalasia with spasm, a new variant characterized by high pressures in the distal esophageal body, occurring in roughly 29% of cases. The utility of this new classification has yet to be definitively shown, although proponents have suggested that it can be used prognostically (type II being more likely to respond to standard therapy than type I or III).
Endoscopy
Several studies have also examined the role of endoscopic ultrasound in diagnosing achalasia.43,44 Although some studies suggest that patients with achalasia may have increased LES and esophageal body wall thickness, these findings are not present in all patients. Currently, endoscopic ultrasound should be considered a research tool for patients with primary achalasia. Its role in secondary achalasia is discussed later.
Treatment
Many important therapeutic principles must be kept in mind before prescribing treatment options for patients with achalasia (Box 18.1). The first is the need to individualize the definition of a treatment threshold because no defined criteria exist. To date, investigators have been unable to define specific criteria for therapy or to evaluate treatment response. This controversy arises from data showing extremely poor correlation between symptoms and radiographic appearance before and after treatment. One more recent study25 comparing the number and the severity of achalasia symptoms with radiographic scores (based on predefined radiographic parameters, such as the degree of esophageal dilation, the LES diameter, and the presence or absence of sigmoidization or retained food debris) found no correlation at all between symptoms and x-ray appearance either before or after treatment. Other studies have had similar results.45,46
Box 18.1 Therapeutic Principles in Achalasia
In addition, one must keep in mind age when evaluating symptoms and treatment response. Studies have shown that older patients with achalasia (>45 to 60 years old depending on the study) tend to have better symptomatic responses to treatment. These improved responses have been shown with botulinum toxin injection47 and with pneumatic dilation.48 Whether the improvement is objective or subjective is unclear because older patients tend to have esophagi that are less sensitive to stimulation49; this population may be less reliable in conveying objective improvement. No outcome trials have been designed as yet to show whether or not early therapeutic intervention leads to an improved clinical outcome.
The second issue is whether one should standardize the criteria used for symptom assessment or radiographic evaluation. There is a wide variation in the way individual physicians take histories from patients with achalasia. Specific symptom scoring methods have been proposed to address this problem to allow standardized evaluation of treatment responses, but they have not been found to be very useful clinically.25,50,51 Similarly, there is great variability in the way a barium swallow is performed in different radiology departments. Difference exists in terms of the amount and consistency of oral contrast medium given, the performance of video techniques versus static images, the use of double-contrast versus single-contrast imaging, and the number and rate of swallows administered.
Some investigators have studied the use of a standardized “timed” barium swallow.45,52,53 The proposed technique requires ingestion of low-density barium sulfate suspension within 30 to 45 seconds (the maximum amount tolerated is measured) with spot films taken at 1 minute, 2 minutes, and 5 minutes. The height and width of the barium column is measured at each time point, and the rate of esophageal emptying from 1 to 5 minutes is recorded (Fig. 18.4).51 In one of the studies using this technique,52 32 patients with near-complete symptom relief after therapy were followed. Initially, there was a significant association between patient symptoms and improvement in barium column height, although esophageal barium emptying was less predictive of the symptomatic response during longer term follow-up. Despite this limitation, patients whose symptom response correlated best with emptying were far less likely to have symptomatic relapse during follow-up.
Fourth, when evaluating the results of therapeutic studies, one must bear in mind that most studies deal with a heterogeneous population of patients with varying degrees of disease severity. Because of significant variability among patients in terms of specific symptoms,25 extrapolating symptom response rates from clinical studies to individual patients may not always be valid. Because of the rarity of this disease, most achalasia studies group patients with all stages of severity together for analysis. These data include therapeutic responses ranging from patients with minimal esophageal dilation and some LES opening to patients with frank sigmoidization, complete aperistalsis, and severe LES spasm. Although the proportion of patients with end-stage disease may be similar among some studies, patients with end-stage disease are often excluded in other studies. In one study evaluating the efficacy of Heller myotomy for achalasia, symptomatic success was related to preoperative disease stage.54 The relatively small numbers of achalasia patients in general also predisposes to type II errors in study design. Finally, the precise details of how therapeutic procedures (botulinum toxin injection, pneumatic dilation, and surgical myotomy) are performed often vary greatly among operators.
Botulinum Toxin Injection
From the innovative work of Pashricha and coworkers,55 botulinum toxin injection into the LES has become a cornerstone of treatment for achalasia. Gastroenterologists have embraced this approach to treatment for three reasons: (1) It is easy to administer, (2) it is safe, and (3) it works. Because of its extremely favorable risk-to-benefit ratio, we commonly use botulinum toxin injection as a temporizing measure in patients presenting with severe disease who are concerned about undergoing more invasive procedures or in patients at poor risk for more invasive procedures.
The precise injection technique is unspecified. In Pashricha’s original work, “the lower esophageal sphincter was visualized endoscopically by identification of the sphincteric rosette typically seen at the squamocolumnar junction. Botulinum toxin was injected through a 5-mm sclerotherapy needle into the region of the lower esophageal sphincter.”55 In a more recent report by Kolbasnik and colleagues,56 botulinum toxin was administered “approximately 5 mm above the Z line.” In a study by Annese and coworkers,57
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree