This article examines real-life case histories of men with routine and not so routine conditions underlying lower urinary tract symptoms (LUTS), and demonstrates the utility of what has become our standard evaluation: repeated bladder diaries, urinary flow rate postvoid residual urine flow, cystoscopy, and videourodynamics, as well as the routinely used LUTS questionnaire. Each case history was sent to each of the other authors of this monograph who, on a case by case basis, answered queries and made relevant comments. The patient evaluations and case histories are discussed by top experts who have authored articles in this issue.
AUASS : American Urologic Association BPH Symptom Score (also known as IPSS)
FBC : Functional bladder capacity, also called MVV – the largest voided volume recorded on a 24 hour bladder diary
MVV : The largest voided volume recorded on a 24 hour bladder diary
NBC index : Nocturnal blader capacity index a measure of nocturnal bladder capacity. The higher the number, the lower the bladder capacity at night compared to daytime bladder capacity
NP index : Nocturnal poluria index = NUV/24 hour urine volume
NUV : Nocturnal urine volume as recorded on a bladder diary
Neurourologic exam : Comprised of anal sphincter tone & control; bulbocavernosus reflex; and perianal sensation
pabd : Abdominal pressure
pdet : Detrusor pressure
pdet@Qmax : Detrusor pressure at maximum uroflow
pves : Vesical pressure
PVR : Post-void residual urine volume
Q : Uroflow
Qmax : Maximum uroflow
SIC : Intermittent self catheterization
UDS : Urodynamic study
USG : Ultrasound
UVJ : Ureterovesical junction
VOID : Shorthand method of depicting Qmax/voided volume/PVR; eg: VOID: 21/355/12 where Qmax = 21; voided volume; 355 and PVR = 12
VUDS : Videourodynamic study
The purpose of this article is to examine real-life case histories of men with routine and not so routine conditions underlying lower urinary tract symptoms (LUTS), and to demonstrate the utility of what has become our standard evaluation: repeated bladder diaries, urinary flow rate ( Q ), postvoid residual urine flow (PVR), cystoscopy, and videourodynamic studies (VUDS). A LUTS questionnaire (see article by Weiss elsewhere in this issue) is also routinely used, but in the interest of brevity, has not been included the questionnaires in the case reports. Each case history was sent to each of the other authors of this monograph who, on a case by case basis, answered queries and made relevant comments.
For years, videourodynamcs and cystoscopy routinely were conducted on all men who required treatment. Those invasive studies are now recommended only under the following circumstances: (1) men who elect invasive therapies, including minimally invasive surgical therapy (MIST); (2) men with hydronephrosis that could be due to outlet obstruction or low bladder compliance; (3) men with repeated very low flow rates and/or high PVR; (4) to confirm or diagnose a neurologic condition; and (5) men who have failed conservative therapies and desire further treatment.
From a physiologic viewpoint, the cause of LUTS is multifactorial, comprising at least 5 conditions: (1) urethral obstruction, (2) impaired detrusor contractility, (3) detrusor overactivity, (4) sensory urgency, and (5) polyuria; it is the purpose of the diagnostic evaluation to define these. Although LUTS have been subdivided into irritative and obstructive bladder symptoms, there is no correlation between these descriptive terms and the underlying physiology (as discussed in more detail in the article by Bushman). Storage symptoms include urinary frequency, urgency, urge incontinence, nocturia, and some kinds of pain. Voiding symptoms include hesitancy, straining, decreased stream, dysuria, and postvoid dribbling. Table 1 lists LUTS and the possible underlying conditions causing each.
| Symptom | Causes |
|---|---|
| Hesitancy weak/interrupted stream | Urethral obstruction |
| Detrusor overactivity | |
| Impaired detrusor contractility | |
| Acquired voiding dysfunction | |
| Urinary frequency | Polyuria |
| Detrusor overactivity | |
| Small capacity bladder | |
| Acquired voiding dysfunction | |
| Low bladder compliance | |
| Urgency urge incontinence | Detrusor overactivity |
| Bladder inflammation/UTI | |
| Stress incontinence | Sphincteric incontinence |
| Stress hyperreflexia | |
| Low bladder compliance | |
| Unaware incontinence | Detrusor overactivity |
| Sphincteric incontinence | |
| Fistula | |
| Nocturia | Nocturnal polyuria |
| Detrusor overactivity | |
| Small capacity bladder | |
| Low nocturnal bladder capacity | |
| Global polyuria (24 h volume >40 mL/kg) | |
| Nocturnal enuresis | Sphincter abnormality |
| Detrusor overactivity | |
| Urinary retention | |
| Postvoid dribble | Postsphincteric collection of urine |
In this article, the patient evaluations and case histories are discussed among top experts who have authored articles in this issue: Dr Wade Bushman, Dr Stephen Petrou, Dr Lori Lerner, Dr Karl Kreder, Dr Charles Powell, Dr Matthew Rutman, Dr Kevin McVary, Dr Robert Donnell, Dr Gary Leach, and Dr Jeffrey Weiss.
Case 1 (KM). Overactive bladder dominated LUTS; treated with medication
Patient: KM is a 49-year-old executive with mixed storage and emptying symptoms.
History: He complains of urinary frequency, urgency, and nocturia and, when queried, admitted that he also has some hesitancy and weak stream, but the overactive bladder (OAB) symptoms dominated the clinical picture. These symptoms began about 6 to 12 months ago and have gradually worsened.
American Urological Association (AUA) symptom score: 27.
Physical examination: Prostate 25 g, smooth; right lobe slightly firmer than left.
Urinalysis : normal.
Questions for the panel: Do you require any more information before initiating treatment?
Answer:
All respondents wanted to see prostate-specific antigen (PSA) level, uroflow, and PVR. Only 40% thought a urine culture and/or urinary cytology was necessary, and 40% wanted to see a bladder diary. Dr McVary wanted to know whether there was a family history of prostate cancer and whether there were any risk factors for urethral stricture. Based on the digital rectal examination (DRE) he said “it sounds like a transrectal ultrasound (TRUS) prostate biopsy should be done.” No one recommended upper tract imaging unless there was an elevated PVR.
Drs Kreder and Powell: “With the urinalysis being negative for leucine esterase and nitrite, we wouldn’t pursue urinary tract infection as the cause but it could still be prostatitis. We would want a PSA so we don’t miss the easy things—prostate cancer and prostatitis. If the PSA is elevated, we would recommend expressed prostatic secretions and culture and a prostate biopsy, especially of the firm area.”
Back to the patient:
Neuro-urologic examination: normal (anal sphincter tone and control, perianal sensation, bulbocavernosus reflex)
Laboratory: PSA .7
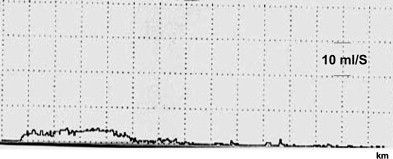
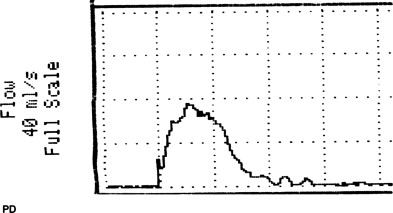
VOID: 4/85/55 (The Q curve is seen in Fig. 1 )
| 24-hour voided volume | 1560 mL |
| Usual voided volume | 90–120 mL |
| Maximum voided volume (MVV) | 210 mL |
| Awake hours: # voids | 8 |
| Sleep hours: # voids | 2 |
| Nocturnal urine volume (NUV) | 330 mL |
| Nocturia index (NUV/MVV) | 1.6 |
| Nocturnal polyuria index (NPi)-NUV/Total 24 hour volume | 15% |
| Nocturnal bladder capacity (NBC) index | 1.4 |
Comment: I consider the neuro-urologic examination—anal sphincter tone and control, perianal sensation, and the bulbocavernosus reflex—to be an essential part of the physical examination. It is almost always normal, but when it is not, it may be the only underlying clue to a neurologic lesion like cauda equina or Shy-Drager syndrome. It takes about 30 to 60 seconds to complete (and your finger is already in the rectum for the DRE)!
And the bladder diary! I don’t know how anyone can treat these patients without one. Look at his. His MVV, which is his functional bladder capacity, is only 210 mL and most of his voids are between 90 and 120 mL. So his volume of 85 mL for the Q is only a bit lower than usual. If his usual voided volumes were 400 mL, for example, we’d more or less ignore this Q and repeat it.
Question for the panel: How would you treat him?
Answer:
Dr Kreder : Start with tamsulosin 0.4 mg every bedtime and have a discussion about finasteride. If he is willing to take another pill and understands the uncertainty some feel about the higher incidence of high-grade cancers coming from the prostate cancer prevention trial, I would start that as well.
Dr Leach : Since his PSA is within normal limits AND Q max is low and PVR low, I would consider a trial of α-blocker.
Dr Donnell : If he wanted treatment (and the PSA, and voiding diary supported), I would use α-blocker as the first line therapy.
Dr Lerner and Dr Bushman : α-Blocker.
Dr McVary : Offer α-blockers, with strict requests for follow-up in office in 6 to 8 weeks.
Dr Powell : I would empirically try α-blockers if PSA is normal. If still not better after 2 months on tamsulosin, would get urodynamic studies (UDS) and consider cystoscopy with repeat urinalysis (UA). He may be a candidate for daily tadalafil if he fails tamsulosin. I would continue tamsulosin.
Dr Rutman : Behavioral modification (fluid and caffeine reduction, bladder retraining), likely selective α-blocker (tamsulosin or uroxatral), possible antimuscarinic if still symptomatic (storage symptoms) despite α-blocker.
Dr Weiss : No treatment until additional information becomes available.
Question for panel: When initiating treatment do you consider any differences between commercially available α-adrenergic medications, that is, is one medication better for this patient than another?
Answer :
Dr Lerner and Dr Bushman: Only that tamsulosin and alfuzosin are easier to use because no need to titrate.
Dr Weiss: Terazosin is cheap, effective, and generally well titrated when begun with a dose pack card. If the patient develops or is at high risk for orthostatic hypotension, tamsulosin is my next line of therapy.
Dr Leach: I think all α-blocker medications are essentially equally efficacious, but generally I prescribe tamsulosin given less orthostatic hypotension and no need to titrate.
Dr Rutman: Yes, I discuss orthostatic hypotension, ejaculatory disturbance. If he is concerned about ejaculation, I would choose alfuzosin over tamsulosin.
Dr McVary: I most commonly use nontitratable α blockers but agree that generics requiring titrations are similar in efficacy.
Dr Kreder: Yes. I feel that tamsulosin is better for older men and those at risk for dizziness and falls; otherwise, all are equally efficacious. If this man had no risk factors, terazosin is my drug of choice because a 100-day supply can be gotten for $19.00 while tamsulosin is $25.03 per 30-day supply, at the least expensive pharmacy Web site. Another difference is that alfuzosin is a good option for a young man with retrograde ejaculation on tamsulosin.
Dr Donnell: I believe that α-blockers are equal, and I prescribe based on patient’s choice.
Back to the patient:
We did not treat him yet. We obtained cystoscopy and videourodynamic study ( Fig. 2 ).
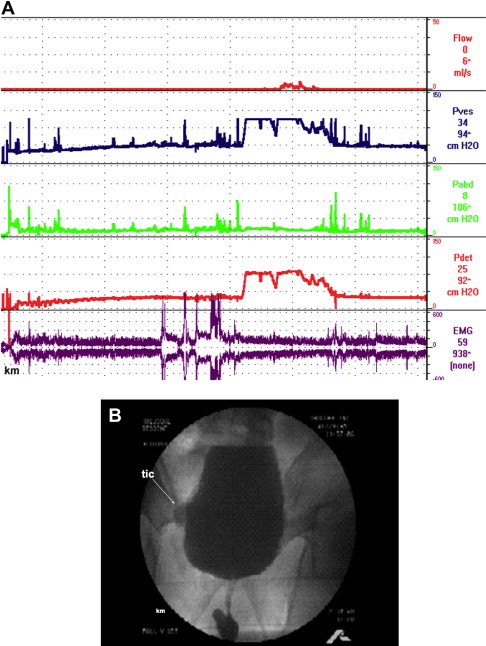
Cystoscopy: minimal bilobar prostatic encroachment, 1+ trabeculation, and small paraureteral bladder diverticulum.
The Schaefer and Abrams-Griffiths nomograms both show significant bladder outlet obstruction ( Fig. 3 A, B ).

Question for panel: Does any of this information change your treatment plan?
Dr Weiss: This man has prostatic obstruction, small bladder capacity, and normal bladder contractility. I would begin an α-blocker, terazosin, titrated initially to 5 mg every bedtime.
Dr Leach : No.
Dr Kreder: Yes. The diverticulum should be carefully examined for tumor, and cytology sent yearly. Fulguration of the diverticulum should be offered, as it offers a minimally invasive way to ablate this small diverticulum. If he begins to develop urinary tract infections (UTIs) then discussion about diverticulectomy should take place. The patient is obstructed and can be started on α-blockers if not already taking them. If he is already on them or has tried them already, then I would offer him transurethral incision of the bladder neck, given the relatively small size of the prostate.
Question: How soon would you see him in follow-up after initiating treatment?
Answer: All respondents said 1 to 3 months.
Question: What efficacy outcomes measures would you use to assess outcomes?
Answer: There was no consensus at all except that 75% of the respondents said they would use the AUA symptom score as part of their assessment. All uses various combinations of symptom and quality of life (QOL) questionnaires, Q , and PVR.
Treatment: Terazosin was begun at 1 mg at bedtime and titrated to 4 mg every bedtime over a 3-month period. He noted a dramatic improvement that has been sustained for 5 years. The most striking difference was, “I only get up once at night (compared with) just about hourly.” He also noted a dramatic decrease in the number and intensity of episodes of urgency and voids with a much better stream. After about a year of treatment, he said “I’m back to normal…I can go to a meeting and I don’t have to get up to urinate.”
Comment: From a clinical perspective, KM was very much improved and very pleased with his treatment. The most striking improvement in symptoms is the marked improvement in urgency, bladder capacity, and nocturia. Although according to his diaries nocturia decreased from 2 times to 1 time per night, he thinks that his first diary was atypical. However, his AUA symptom score only improved from 27 to 18, which is still considered to be a moderately severe symptom score. This points out the importance of not relying on objective criteria alone. Diaries and symptoms scores are very useful, but only when they are put into clinical perspective by talking to the patient.
Objectively, his improvement was manifest by an increase in bladder capacity from 210 to 410 mL. However, his urinary stream is not very different than when he began treatment, and objectively, his uroflow remains quite low and has a long plateau shape, consistent with his documented prostatic urethral obstruction (Table 2) .
| Date | Terazosin Dose (mg) | AUA Score | No. of Day Voids | No. of Night Voids | Bladder Capacity | Usual Voided Volume | Q max | Voided Volume | PVR |
|---|---|---|---|---|---|---|---|---|---|
| 11/94 | 0 | 27 | 7 | 2 | 210 | 90–120 | 4 | 85 | 55 |
| 2/95 | 3 | 10 | 1 | 250 | 7 | 62 | |||
| 3/95 | 4 | 7 | 230 | 83 | |||||
| 8/95 | 4 | 22 | 8 | 0 | 460 | 130–165 | 10 | 289 | |
| 10/96 | 7 | 19 | 10 | 1 | 350 | 130–230 | 7 | 304 | 93 |
| 11/97 | 9 | 8 | 0 | 240 | 140–230 | 9 | 355 | 19 | |
| 7/99 | 5 | 18 | 9 | 1 | 410 | 160–200 | 4 | 398 | 149 |
So, what got better? It is doubtful that his obstruction is improved. If anything, his most recent uroflow suggests that it is either worse, or he has developed impaired detrusor contractility. The only way of finding out is to repeat his videourodynamic studies (VUDS), but this was not done because from a symptomatic viewpoint he felt so much better. Serial renal and bladder ultrasounds were done in KM to be sure that his voiding pattern was not causing bladder or renal complications. His bladder diverticulum appears unchanged and, aside from a benign appearing renal cyst, the kidneys remained normal.
Why did his bladder capacity improve? We don’t know, but there were many comments from the panel.
Dr Lerner: It has been suggested, by Cucci I believe, that development of frequency as a function of diminished bladder compliance is a compensatory response of the detrusor to increased outlet resistance that is done to make bladder emptying more energy efficient. It may be simply that α-blockers interrupt this compensatory response.
Dr Weiss: I agree with the aforementioned. There may be a “clinic” effect that amounts to placebo benefit from generally being followed in a genitourinary office. Just doing voiding diaries can change drinking and voiding behavior to result in the perception of improvement from treatment received.
Dr Leach: An obvious possibility could be the chronic distension from increasing residuals.
Dr McVary: From a urodynamic perspective, α-adrenergic blockade in patients with urodynamically proven obstruction receiving terazosin causes a significant decrease in urethral resistance at 26 weeks of therapy compared with none of the patients without obstruction. However, the actual improvement in urodynamic parameters is less impressive than improvement in free urinary flow and symptoms using the international prostate symptom score. Further studies are needed to identify reasons for the symptomatic benefit gleaned from α-blockers out of proportion to improvement in urodynamic parameters of pressure flow and urethral resistance factors.
Dr Petrou: I don’t know that it really matters, since there’s no compelling evidence that unrelieved obstruction moves inexorably to worsening symptoms or acute urinary retention. If the symptoms are relieved—that’s enough. However, I would have offered the man a transurethral incision of the prostate (TUIP) as a very good alternative. A 1-hour procedure with minimal morbidity would be likely to provide even greater symptomatic improvement and obviate a lifetime of medication.
Dr Weiss: As long as the patient is happy, with no worrisome symptoms such as worsening urgency or development of incontinence and the PVR is not rising, I would continue indefinitely with conservative therapy. The only long-term potential problem I know of from the use of chronic α-blockers is the effect on the eye, especially with respect to the risk of complications from cataract surgery.
Dr Leach: We know that α-blockers may affect bladder function by changing outflow resistance or can possibly have a direct effect on bladder function. A urodynamic study would have been helpful in determining the etiology of the change in capacity.
Dr Kreder: α-Blockers have been shown to increase bladder capacity in one abstract presented at a recent International Continence Society meeting that comes to mind. The investigators used endorectal ultrasound to assess blood flow to the bladder and prostate as well as bladder capacity before and after 5 weeks of tamsulosin. The bladder was filled with NaCl followed by KCl to mimic irritative symptoms. The maximum urodynamic bladder capacity increased by 100 mL for the KCl group treated with tamsulosin. There was no change for the NaCl group. No mechanism was proposed for this effect, other than the observation that blood flow was increased significantly by tamsulosin. This would be consistent with the findings in this case.
Case 2 (PD). Overactive bladder predominant LUTS treated with surgery
Patient: PD is a 56-year-old insurance broker with OAB predominant LUTS.
Urologic history: His chief complaint is urinary frequency, urgency, and nocturia of over 10 years’ duration. He voids more often than once an hour during the day and has nocturia about every 1 to 2 hours at night. He has never been incontinent, but “I’ve been close.” He always has hesitancy and a weak stream. He is currently being treated with tamsulosin, 4 mg twice a day and finasteride, 5 mg every day. PSA has ranged from 6.3 to 7 for the last 5 years, and he has had 2 sextant prostate biopsies that have shown benign prostate hyperplasia (BPH). The last biopsy was 6 months ago.
Prior treatment: doxazosin (4 mg every day), terazosin (every day). Each was discontinued because of lightheadedness.
Medical history: mild asthma.
AUA symptom score: 27.
Physical examination: Prostate estimated 15 to 20 g in size, normal consistency, and no nodules.
Laboratory: Total PSA = 3.30 mg/mL.
Free PSA = 0.49 mg/mL (15%).
Urinalysis = normal.
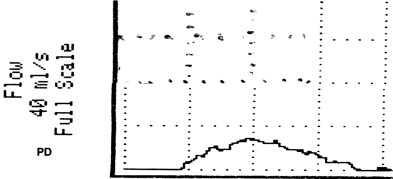
VOID: 7/62/20 Fig. 4
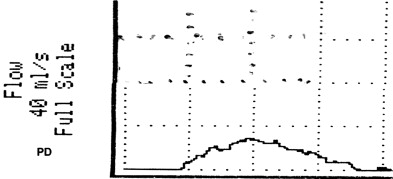
Questions for the panel: It’s been 6 months since his last biopsy; would you recommend another or more aggressive biopsy? Anything else to aid in the diagnosis of prostate cancer?
Answer:
Dr Weiss: Yes. Our recent study of a large database of men undergoing prostate biopsy concluded that the ideal ratio of prostate volume/core ratio to maximize the diagnosis of prostate cancer is 3 mL/core biopsy. Assuming his prostate is actually 20 mL, a sextant biopsy (and even better, 2 of these sessions) should adequately cover such a gland. His PSA has appropriately come down in response to finasteride, from between 6 and 7 to 3.3. However, the elevated PSA on its own mitigates saturation biopsies, which in this case would be 12 in all.
Dr Petrou: Since the diagnosis of prostate cancer is an issue in view of the history of finasteride and his free PSA total, then I would ask him to undergo a transperineal ultrasound guided templated prostate biopsies. This would also be an ideal way of obtaining a very accurate prostate volume if further therapy is being considered.
Dr Lerner: First, I’d like to make sure that his urinalysis is negative and that his OAB symptoms aren’t related to hematuria/bladder cancer. Second, what was the prostate size on TRUS? Is that the 15 to 20 g? Third, has he had any response at all to meds? Did they improve his symptoms, even a little bit? I think this is important to know because it helps us know if this is a BPH/bladder outflow obstruction (BOO) kind of picture versus a high bladder neck. Fourth, did they see inflammation on the biopsies consistent with prostatitis, which could explain his PSA? Lastly, is there a family history of prostate cancer and/or Agent Orange exposure or something that makes us worry about prostate cancer other than the PSA?
Dr Rutman: I would tell him that sextant biopsies are not the standard of care in 2009. Considering he is on finasteride (Proscar) and his PSA is significantly elevated with a palpably small gland, I would recommend a 12-core biopsy if he was interested in being screened for prostate cancer.
Dr Leach: Yes, I would repeat prostate biopsy (6 times each side).
Dr Donnell : In light of his young age, the elevated PSA, the decreased percent free PSA, the reported small prostate size without evidence of inflammation on biopsy, I would repeat a more aggressive biopsy. From this information I would also confirm the prostate size by ultrasound. The diagnosis of prostate cancer would certainly change my approach to his LUTS over the near future.
Question: Assuming that he does not have prostate cancer and he does not want surgery, would you continue to treat him empirically or do you want more studies (urodynamics, cystoscopy)?
Dr Weiss: If his urinalysis and cytology are normal, cystoscopy is optional although I find it useful and would recommend it. The next step before UDS is a voiding diary, preferably several. This will tell us what the functional bladder capacity would be and whether some extraurologic condition such as global polyuria or nocturnal polyuria are contributing to his symptoms. In view of the long-standing nature of his symptoms, urodynamics are indicated as they are in anyone with near-urgency incontinence.
Dr Lerner: It would depend on his response to meds. If he is not responding well to meds, I would encourage a pressure flow study.
Dr Rutman: If he is not interested in surgery and wishes to proceed with medical therapy, I would not perform urodynamics. I would only perform cystoscopy if he had microscopic hematuria.
Dr Donnell : Assuming that he does not have prostate cancer, a urinalysis, cytology, and voiding diary are acceptable. I would then proceed with urodynamics.
Dr Leach: I would do urodynamics and cystoscopy.
Dr Petrou: I would do cystoscopy, urodynamics, and urine cytology.
Question: Assuming that he does not have prostate cancer, and he does not want surgery, how would you treat him now?
Dr Weiss: I would not treat such a patient empirically. He has had quite enough of that for years. Treatment would be tailored to the most specific diagnosis I could derive from analysis of the voiding diary, cystoscopy, and urodynamic study.
Dr Lerner: Urinary urgency always concerns me regarding bladder compensation/decompensation. If he didn’t want surgery, I would encourage him to make sure that his bladder wasn’t “asking for help.” I would consider anticholinergics, but his age suggests that if obstruction were found, a lot of his instability could resolve after treatment.
Dr Rutman: I would add anticholinergics to treat his storage symptoms. Since he also has obstructive symptoms, I would use an α-blocker in addition. If he did not have obstructive symptoms, I would use the anticholinergic alone. I do not believe there is a significant difference between anticholinergic agents as a class, with the caveat that some agents work better in some patients in a completely unpredictable fashion.
Dr Leach: His diagnosis is bladder outlet obstruction and probable detrusor overactivity. With a small prostate (20 g) I would discontinue finasteride and continue tamsulosin, and add an anticholingeric (darifenacin 7.5 mg every bedtime).
Dr Petrou: I’d recommend combined therapy with α-blockers and anticholinergics.
Question: If you recommend anticholinergics, do you think it is necessary to always use α-blockers as well? Do you think there are significant differences among anticholinergics?
Answers:
Dr Petrou: Single-agent initial therapy is acceptable; significant differences are an active point of debate among many of our colleagues, although if the patient does have some postvoid residual present, trospium chloride may be of value in view of its unmetabolized direct effect on the urothelium. Of attractiveness for the contemporary male patient may be the topical gel anticholinergics, in view of the non-pill format and the acceptance of hormone replacement in this fashion.
Dr Weiss: If I’m not sure if a man is obstructed I tend to start an α-blocker as “pretreatment” before institution of anticholinergics. While different patients respond in variable fashion at times to available anticholinergics, we do not have the ability to predict which patients will respond best to which of these. The theoretical differences in receptor affinity among the newer anticholinergics do not translate into major predictable clinical responses. (Perhaps in the elderly one might consider trospium, as it crosses the blood-brain barrier less well than all others owing to its status as a charged amine.) For this reason, and for reasons of economy, assuming there is no history of narrow-angle glaucoma I begin with oxybutynin at a low dose, 2.5 mg twice a day, then have them return in 3 to 4 weeks with a fresh diary for Q , PVR, and questionnaire.
Dr Lerner: No, I do not always think it is necessary to use an α-blocker with an anticholinergic. Yes, there are some differences between drugs, but some patients just do better with one drug over another. I start with oxybutynin, then go to tolterodine, then onwards from there. I tend to avoid the long-acting meds, simply because I don’t think they work as well in obstruction-related instability. Neurogenics and age-related instability may do well with long acting, but I have anecdotally found that the peaks of shorter-acting meds are more efficacious.
Dr Leach: I would not give an anticholinergic without the α-blocker in an obstructed man with BPH. I prefer darifenacin due to the lack of “cortical” side effects (memory loss and so forth).
Dr Bushman : I would start anticholinergics along with the α-blocker, establishing the response, and then remove the α-blocker.
Dr Donnell : If I recommend an anticholinergic I refer to the presence or absence of my urodynamics. I will empirically start an α-blocker before an anticholinergic. I do believe that there are differences between the drugs, but for the majority of patients there is little difference in efficacy and a larger concern for side effects.
Question: What is the role for MIST in a patient who says that he doesn’t want surgery? Do you consider MIST to be “surgery?” What do you tell the patient?
Dr Weiss: I have been personally disappointed with nonvaporizing treatments that heat the prostate, and do not use or recommend them. They certainly are capable of causing catastrophic complications such as severe bladder neck stenosis. They are inequitably (compared with transurethral resection of the prostate [TURP], TUIP, laser prostatectomy) well compensated by third-party carriers, which in my view is the main reason they persist in urologic practice.
Dr Lerner: I NEVER do MIST, ever.
Dr Rutman: I would discuss all options including Prostiva radiofrequency ablation (RFA) and Microwave, although I am not a huge advocate of these procedures. I would still inform him of the options and refer him if that was a strong consideration. I consider the RFA to be the better option of the two, but I would let him know of all potential outcomes including worsening of his storage symptoms, retention, and lack of efficacy. Since the patient has a small prostate he may be amenable to a TUIP. Otherwise he would be a candidate for any intervention to relieve his obstruction.
Dr Leach: I would not do MIST in a man with OAB symptoms. In my experience, if they get any relief from their OAB symptoms, it is only temporary and they usually get worse before they get better.
Dr Petrou: The larger the volume the less inclination for minimally invasive technique. All surgery including minimally invasive techniques are major surgeries to the person receiving the therapy.
Dr Donnell: MIST therapy can certainly play a role for the patient who does not want surgery and even for that patient who does not want to take pills. I share with my patients that transurethral needle ablation (TUNA) and transurethral microwave therapy (TUMT) are procedures requiring a well-performed regional bloc of the prostate and oral pain medications. While not surgery, I share with them that their time commitment and recovery effort the first day are similar to regular surgery.
Question: How does prostate size influence your treatment recommendations?
Dr Weiss: Men with larger, more glandular prostates may respond better to 5α-reductase inhibitors, especially when the PSA is slightly elevated above normal. I favor KTP laser prostatectomy for small to medium-size prostates and TURP for larger glands. For prostates over 100 mL it is reasonable to consider open prostatectomy, especially in the presence of bladder stones.
Dr Lerner: Size makes a difference between a TUIP versus a more complete trilobar surgery.
Dr Rutman: I would recommend urodynamics to evaluate his bladder function. If he had good bladder function, I would recommend an outlet reductive procedure such as a TUIP or a Green Light HPS Laser prostatectomy.
Dr Leach: Only in that I would not use finasteride with a prostate less than 50 g in size.
Dr Donnell : We do need to be aware of prostate size due to the technical limitations of these particular therapies. An ultrasound would provide information concerning prostate volume, the presence of a median lobe with intravesicle protrusion, which appear to be important parameters associated with increased failure of minimally invasive therapies. Certainly there is a size relationship with TUIP and 5α-reductase inhibitors. There also appears to be a size relationship with Botox, TUNA, and TUMT. Early evidence would support that Botox is possibly more appropriate in the smaller gland as would be TUNA, while TUMT may be better served in that patient with a larger prostate.
Question: If he says that he will follow your advice and do whatever treatment you think will achieve the best outcome, what is your advice?
Dr Weiss: I don’t have enough data at this point to recommend a treatment pathway. I want to see the results of the voiding diary, cystoscopy, and urodynamic study.
Dr Lerner and Dr Donnell: I would do UDS and then decide.
Dr Rutman: If we were going to prescribe an antimuscarinic agent, I’d see him back 4 to 6 weeks after instituting therapy. If there was any concern about increasing retention, maybe sooner to check a postvoid residual (2 weeks after starting the antimuscarinic).
Dr Leach: I suggest an α-blocker combined with an anticholinergic agent at this point.
Back to the patient:
| 24-hour voided volume | 1,500 mL |
| Awake hours: # voids | 9 |
| Sleep hours: # voids | 4 |
| Usual voided volume | 120 mL |
| Functional bladder capacity | 240 mL |
| Nocturnal urinary volume | 480 mL |
| NUV/Total | 32% |
| NBC/Index | 3 |
| Nocturia index (NUV/FBC) | 2 |
Cystoscopy: The prostatic urethra was over 6 cm in length and there was a very large intravesical lobe. The bladder was 4+ trabeculated.
Urodynamic study: (8 April, 1999) Tracing is depicted in Fig. 5
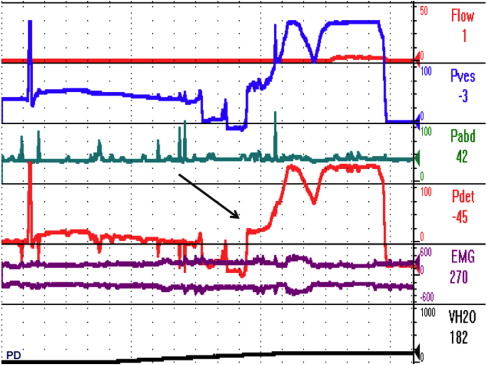
Fig. 6 A, B depict the bladder during filling and voiding.
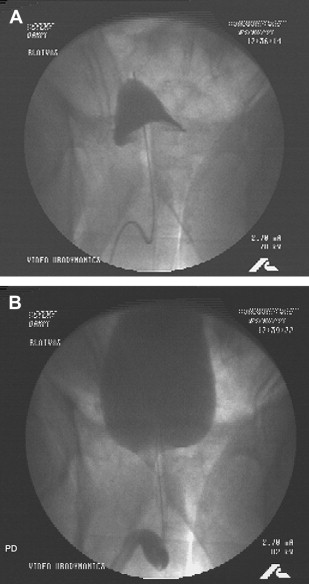
Urodynamic diagnosis:
- 1.
Prostatic obstruction
- 2.
Type 3 OAB (detrusor overactivity)
Repeat sextant TRUS prostate biopsy showed nodular BPH without signs of chronic inflammation. The prostate was estimated to be about 80 g in size.
Question: How would you treat this patient? Please describe how you came to your decision.
Dr Weiss: With the large gland and intravesical component, suprapubic prostatectomy is the treatment of choice although many skilled resectionists would feel TURP may be done safely with a similar outcome while avoiding an incision. KTP laser prostatectomy is safe and effective in the hands of those accustomed to performing this procedure on large prostates, and may be the treatment of choice for men on anticoagulant therapy, even if it means staged procedures would be necessary.
Dr Lerner: I would have done UDS early in this man because of his failure to respond to meds. Then, I would have recommended holmium laser enucleation of the prostate. I had a feeling that his TRUS volume was different than that of his DRE. If BOO is present, then surgery is where I go next. Anticholinergics may be necessary during the postoperative recovery phase, and I use them often.
Dr Rutman: This case played out differently than I expected since the prostate volume was estimated at 15 mL on physical examination. I would have likely offered the patient a Green Light Laser prostatectomy, bipolar TURP, and an open prostatectomy (if the median lobe was gigantic and essentially unresectable transurethrally). I would not recommend a MIST for a patient with a large gland.
Dr Leach: I would also do an open prostatectomy.
Dr Petrou: Single medical therapy followed by multimodal therapy, then followed by tissue ablative therapy if needed.
Dr Donnell : The final outcome of this patient would not warrant a MIST therapy at the time of his presentation for TURP. I would offer him a high-power laser ablation. One would question whether the minimally invasive therapies would have altered his final presentation in either the final presenting symptoms or the time course of the disease. With the HPS laser system the number of open prostatectomies I perform has decreased dramatically.
Back to the patient:
Treatment: Suprapubic prostatectomy was performed without incident. Pathology was nodular BPH (no inflammation).
Follow-up: (4 weeks postop): He said he was voiding with a better stream than he could ever remember, but was disappointed that he still had OAB symptoms and was most bothered by the persistent nocturia.
VOID: 19/207/52 ( Fig. 7 ).
| 24-hour voided volume | 1,620 mL |
| Daytime urinary volume | 900 mL |
| Nocturnal urinary volume | 720 mL |
| Awake hours: # voids | 8 |
| Sleep hours: # voids | 3 |
| Usual voided volume | 120 mL |
| MVV | 240 mL |
| NP index | 44% |
| NBC index | 1 |
| Nocturia index (NUV/MVV) | 3 |
The diary showed persistence of nocturnal polyuria He was started on a low-key behavioral program with particular emphasis on cutting back on fluid intake after his nighttime meal, and made considerable improvement. A bladder diary 6 months postop showed complete resolution of his nocturnal polyuria and OAB symptoms. In addition, his functional bladder capacity increased to 350 mL. He said “I can’t believe that I had to wait 5 years for this; I’m cured.”
Voiding diary: 6 months postop
| 24-hour voided volume | 1750 mL |
| Awake hours: # voids | 4 |
| Sleep hours: # voids | 2 |
| Usual voided volume | 300 mL |
| MVV | 350 mL |
| NUV | 600 mL |
| NP index | 33% |
| NBC index | 2 |
| Nocturia index (NUV/FBC) | 2 |
He was unchanged at 1 year follow-up and never returned to the office, but responded to a telephone interview and said that he remains “cured.” He declined further prostate cancer screening.
Discussion: PD has had over a decade of unsuccessful “conservative therapy” for BPH. He’d failed almost all of the commercially available α-adrenergic agonists and had at various times been advised to undergo a variety of laser, ultrasonic, and other thermal procedures intended to accomplish prostatic ablation. His symptoms began when he was in his early forties, and he was thought to be “too young” to have prostatic urethral obstruction.
He consulted us about treatment options. Although his PSA had fallen to 3.3, he was being treated with finasteride and his free PSA was 15%. Repeat sextant biopsy was done to rule out prostate cancer. Prostate ultrasound estimated the size of his prostate at only 80 g, but because of the length of the prostatic urethra (>6 cm) and the large intravesical component, open prostatectomy was elected. At the time none of the current laser surgeries were available.
From our perspective, the choice between TURP, laser prostatic ablation, and open prostatectomy is a personal one based primarily on the preference of the surgeon (hopefully he or she is a reasonable judge of his or her surgical skills).
We believe that the choice between a retropubic and suprapubic approach is best made at the time of surgery. If there is sufficient prostatic bulk to accomplish an incision in the anterior prostatic capsule, we prefer a retropubic approach because we believe that it is easier to control bleeding. However, if most of the prostate is intravesical, as it was in this case, a suprapubic approach is technically easier.
For the first month or so his storage symptoms (frequency, urgency, and nocturia) were unabated despite a marked improvement in flow from 7 to 19 mL/s, but his most bothersome symptoms (nocturia) was due to a nonurologic condition—nocturnal polyuria—and was effectively treated by behavior modification. By 6 months postop he was totally asymptomatic.
There is, of course, a concern about prostate cancer, given his PSA history, but we respect his decision to decline further screening.
Case 2 (PD). Overactive bladder predominant LUTS treated with surgery
Patient: PD is a 56-year-old insurance broker with OAB predominant LUTS.
Urologic history: His chief complaint is urinary frequency, urgency, and nocturia of over 10 years’ duration. He voids more often than once an hour during the day and has nocturia about every 1 to 2 hours at night. He has never been incontinent, but “I’ve been close.” He always has hesitancy and a weak stream. He is currently being treated with tamsulosin, 4 mg twice a day and finasteride, 5 mg every day. PSA has ranged from 6.3 to 7 for the last 5 years, and he has had 2 sextant prostate biopsies that have shown benign prostate hyperplasia (BPH). The last biopsy was 6 months ago.
Prior treatment: doxazosin (4 mg every day), terazosin (every day). Each was discontinued because of lightheadedness.
Medical history: mild asthma.
AUA symptom score: 27.
Physical examination: Prostate estimated 15 to 20 g in size, normal consistency, and no nodules.
Laboratory: Total PSA = 3.30 mg/mL.
Free PSA = 0.49 mg/mL (15%).
Urinalysis = normal.
VOID: 7/62/20 Fig. 4
Questions for the panel: It’s been 6 months since his last biopsy; would you recommend another or more aggressive biopsy? Anything else to aid in the diagnosis of prostate cancer?
Answer:
Dr Weiss: Yes. Our recent study of a large database of men undergoing prostate biopsy concluded that the ideal ratio of prostate volume/core ratio to maximize the diagnosis of prostate cancer is 3 mL/core biopsy. Assuming his prostate is actually 20 mL, a sextant biopsy (and even better, 2 of these sessions) should adequately cover such a gland. His PSA has appropriately come down in response to finasteride, from between 6 and 7 to 3.3. However, the elevated PSA on its own mitigates saturation biopsies, which in this case would be 12 in all.
Dr Petrou: Since the diagnosis of prostate cancer is an issue in view of the history of finasteride and his free PSA total, then I would ask him to undergo a transperineal ultrasound guided templated prostate biopsies. This would also be an ideal way of obtaining a very accurate prostate volume if further therapy is being considered.
Dr Lerner: First, I’d like to make sure that his urinalysis is negative and that his OAB symptoms aren’t related to hematuria/bladder cancer. Second, what was the prostate size on TRUS? Is that the 15 to 20 g? Third, has he had any response at all to meds? Did they improve his symptoms, even a little bit? I think this is important to know because it helps us know if this is a BPH/bladder outflow obstruction (BOO) kind of picture versus a high bladder neck. Fourth, did they see inflammation on the biopsies consistent with prostatitis, which could explain his PSA? Lastly, is there a family history of prostate cancer and/or Agent Orange exposure or something that makes us worry about prostate cancer other than the PSA?
Dr Rutman: I would tell him that sextant biopsies are not the standard of care in 2009. Considering he is on finasteride (Proscar) and his PSA is significantly elevated with a palpably small gland, I would recommend a 12-core biopsy if he was interested in being screened for prostate cancer.
Dr Leach: Yes, I would repeat prostate biopsy (6 times each side).
Dr Donnell : In light of his young age, the elevated PSA, the decreased percent free PSA, the reported small prostate size without evidence of inflammation on biopsy, I would repeat a more aggressive biopsy. From this information I would also confirm the prostate size by ultrasound. The diagnosis of prostate cancer would certainly change my approach to his LUTS over the near future.
Question: Assuming that he does not have prostate cancer and he does not want surgery, would you continue to treat him empirically or do you want more studies (urodynamics, cystoscopy)?
Dr Weiss: If his urinalysis and cytology are normal, cystoscopy is optional although I find it useful and would recommend it. The next step before UDS is a voiding diary, preferably several. This will tell us what the functional bladder capacity would be and whether some extraurologic condition such as global polyuria or nocturnal polyuria are contributing to his symptoms. In view of the long-standing nature of his symptoms, urodynamics are indicated as they are in anyone with near-urgency incontinence.
Dr Lerner: It would depend on his response to meds. If he is not responding well to meds, I would encourage a pressure flow study.
Dr Rutman: If he is not interested in surgery and wishes to proceed with medical therapy, I would not perform urodynamics. I would only perform cystoscopy if he had microscopic hematuria.
Dr Donnell : Assuming that he does not have prostate cancer, a urinalysis, cytology, and voiding diary are acceptable. I would then proceed with urodynamics.
Dr Leach: I would do urodynamics and cystoscopy.
Dr Petrou: I would do cystoscopy, urodynamics, and urine cytology.
Question: Assuming that he does not have prostate cancer, and he does not want surgery, how would you treat him now?
Dr Weiss: I would not treat such a patient empirically. He has had quite enough of that for years. Treatment would be tailored to the most specific diagnosis I could derive from analysis of the voiding diary, cystoscopy, and urodynamic study.
Dr Lerner: Urinary urgency always concerns me regarding bladder compensation/decompensation. If he didn’t want surgery, I would encourage him to make sure that his bladder wasn’t “asking for help.” I would consider anticholinergics, but his age suggests that if obstruction were found, a lot of his instability could resolve after treatment.
Dr Rutman: I would add anticholinergics to treat his storage symptoms. Since he also has obstructive symptoms, I would use an α-blocker in addition. If he did not have obstructive symptoms, I would use the anticholinergic alone. I do not believe there is a significant difference between anticholinergic agents as a class, with the caveat that some agents work better in some patients in a completely unpredictable fashion.
Dr Leach: His diagnosis is bladder outlet obstruction and probable detrusor overactivity. With a small prostate (20 g) I would discontinue finasteride and continue tamsulosin, and add an anticholingeric (darifenacin 7.5 mg every bedtime).
Dr Petrou: I’d recommend combined therapy with α-blockers and anticholinergics.
Question: If you recommend anticholinergics, do you think it is necessary to always use α-blockers as well? Do you think there are significant differences among anticholinergics?
Answers:
Dr Petrou: Single-agent initial therapy is acceptable; significant differences are an active point of debate among many of our colleagues, although if the patient does have some postvoid residual present, trospium chloride may be of value in view of its unmetabolized direct effect on the urothelium. Of attractiveness for the contemporary male patient may be the topical gel anticholinergics, in view of the non-pill format and the acceptance of hormone replacement in this fashion.
Dr Weiss: If I’m not sure if a man is obstructed I tend to start an α-blocker as “pretreatment” before institution of anticholinergics. While different patients respond in variable fashion at times to available anticholinergics, we do not have the ability to predict which patients will respond best to which of these. The theoretical differences in receptor affinity among the newer anticholinergics do not translate into major predictable clinical responses. (Perhaps in the elderly one might consider trospium, as it crosses the blood-brain barrier less well than all others owing to its status as a charged amine.) For this reason, and for reasons of economy, assuming there is no history of narrow-angle glaucoma I begin with oxybutynin at a low dose, 2.5 mg twice a day, then have them return in 3 to 4 weeks with a fresh diary for Q , PVR, and questionnaire.
Dr Lerner: No, I do not always think it is necessary to use an α-blocker with an anticholinergic. Yes, there are some differences between drugs, but some patients just do better with one drug over another. I start with oxybutynin, then go to tolterodine, then onwards from there. I tend to avoid the long-acting meds, simply because I don’t think they work as well in obstruction-related instability. Neurogenics and age-related instability may do well with long acting, but I have anecdotally found that the peaks of shorter-acting meds are more efficacious.
Dr Leach: I would not give an anticholinergic without the α-blocker in an obstructed man with BPH. I prefer darifenacin due to the lack of “cortical” side effects (memory loss and so forth).
Dr Bushman : I would start anticholinergics along with the α-blocker, establishing the response, and then remove the α-blocker.
Dr Donnell : If I recommend an anticholinergic I refer to the presence or absence of my urodynamics. I will empirically start an α-blocker before an anticholinergic. I do believe that there are differences between the drugs, but for the majority of patients there is little difference in efficacy and a larger concern for side effects.
Question: What is the role for MIST in a patient who says that he doesn’t want surgery? Do you consider MIST to be “surgery?” What do you tell the patient?
Dr Weiss: I have been personally disappointed with nonvaporizing treatments that heat the prostate, and do not use or recommend them. They certainly are capable of causing catastrophic complications such as severe bladder neck stenosis. They are inequitably (compared with transurethral resection of the prostate [TURP], TUIP, laser prostatectomy) well compensated by third-party carriers, which in my view is the main reason they persist in urologic practice.
Dr Lerner: I NEVER do MIST, ever.
Dr Rutman: I would discuss all options including Prostiva radiofrequency ablation (RFA) and Microwave, although I am not a huge advocate of these procedures. I would still inform him of the options and refer him if that was a strong consideration. I consider the RFA to be the better option of the two, but I would let him know of all potential outcomes including worsening of his storage symptoms, retention, and lack of efficacy. Since the patient has a small prostate he may be amenable to a TUIP. Otherwise he would be a candidate for any intervention to relieve his obstruction.
Dr Leach: I would not do MIST in a man with OAB symptoms. In my experience, if they get any relief from their OAB symptoms, it is only temporary and they usually get worse before they get better.
Dr Petrou: The larger the volume the less inclination for minimally invasive technique. All surgery including minimally invasive techniques are major surgeries to the person receiving the therapy.
Dr Donnell: MIST therapy can certainly play a role for the patient who does not want surgery and even for that patient who does not want to take pills. I share with my patients that transurethral needle ablation (TUNA) and transurethral microwave therapy (TUMT) are procedures requiring a well-performed regional bloc of the prostate and oral pain medications. While not surgery, I share with them that their time commitment and recovery effort the first day are similar to regular surgery.
Question: How does prostate size influence your treatment recommendations?
Dr Weiss: Men with larger, more glandular prostates may respond better to 5α-reductase inhibitors, especially when the PSA is slightly elevated above normal. I favor KTP laser prostatectomy for small to medium-size prostates and TURP for larger glands. For prostates over 100 mL it is reasonable to consider open prostatectomy, especially in the presence of bladder stones.
Dr Lerner: Size makes a difference between a TUIP versus a more complete trilobar surgery.
Dr Rutman: I would recommend urodynamics to evaluate his bladder function. If he had good bladder function, I would recommend an outlet reductive procedure such as a TUIP or a Green Light HPS Laser prostatectomy.
Dr Leach: Only in that I would not use finasteride with a prostate less than 50 g in size.
Dr Donnell : We do need to be aware of prostate size due to the technical limitations of these particular therapies. An ultrasound would provide information concerning prostate volume, the presence of a median lobe with intravesicle protrusion, which appear to be important parameters associated with increased failure of minimally invasive therapies. Certainly there is a size relationship with TUIP and 5α-reductase inhibitors. There also appears to be a size relationship with Botox, TUNA, and TUMT. Early evidence would support that Botox is possibly more appropriate in the smaller gland as would be TUNA, while TUMT may be better served in that patient with a larger prostate.
Question: If he says that he will follow your advice and do whatever treatment you think will achieve the best outcome, what is your advice?
Dr Weiss: I don’t have enough data at this point to recommend a treatment pathway. I want to see the results of the voiding diary, cystoscopy, and urodynamic study.
Dr Lerner and Dr Donnell: I would do UDS and then decide.
Dr Rutman: If we were going to prescribe an antimuscarinic agent, I’d see him back 4 to 6 weeks after instituting therapy. If there was any concern about increasing retention, maybe sooner to check a postvoid residual (2 weeks after starting the antimuscarinic).
Dr Leach: I suggest an α-blocker combined with an anticholinergic agent at this point.
Back to the patient:
| 24-hour voided volume | 1,500 mL |
| Awake hours: # voids | 9 |
| Sleep hours: # voids | 4 |
| Usual voided volume | 120 mL |
| Functional bladder capacity | 240 mL |
| Nocturnal urinary volume | 480 mL |
| NUV/Total | 32% |
| NBC/Index | 3 |
| Nocturia index (NUV/FBC) | 2 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








