Bladder diverticula are common enough to be encountered by most urologists in practice but are reported less frequently in the literature than they were 50 years ago. Some patients can be managed nonoperatively, whereas others will need surgical intervention consisting of bladder outlet reduction and possibly removal of the diverticulum itself. In addition to the decision to operate, the timing of each intervention deserves careful consideration. Cystoscopy, computed tomography with contrast, urodynamic studies, cytology, and voiding cystourethrography play important roles in informing the clinician. Many new techniques for treatment of the bladder outlet and the diverticulum are available, such as laparoscopy and robotic surgery.
Ever since Rokitansky noted that bladder diverticula resulted from urethral obstruction in 1849, urologists have associated the treatment of the bladder outlet with the treatment of diverticula, but until 1906 there were only 5 cases of bladder diverticula described in the American literature. Large series on bladder diverticula are not reported in the literature as commonly as they were in the first part of the twentieth century. The largest contemporary series from the last 10 years reports on 25 patients, and studies from 1940 to 1967 describe as many as 285 patients. This may lead one to speculate that the problem is not as prevalent, or that interest for this clinical entity in the scientific community has waned. The authors believe diverticula of the bladder have become less prevalent in North America, perhaps because of more treatment options for benign prostatic hyperplasia (BPH) and a tendency to recognize and treat BPH earlier. Therefore, a review of the available data is important for the modern practicing clinician, who may not see the condition frequently. The authors’ experience, lessons from contemporary literature, and experience from earlier literature are examined to provide a background for the modern clinician to make informed treatment decisions. Many exciting new approaches to diverticula have been described recently, such as improved imaging technology and urodynamic studies to guide the decision to perform bladder outlet-reducing surgery and better plan the surgical approach. New surgical approaches involving laparoscopy and robotic surgery have also become available. However, the principles guiding the decision to operate remain the same.
Etiology and incidence
There are 2 categories of bladder diverticula: congenital diverticula, which present primarily in childhood; and acquired diverticula, which are typically seen in adulthood.
Congenital diverticula usually present in boys less than 10 years of age, and the incidence is estimated to be 1.7%. These are believed to be caused by insufficient muscle backing of the ureterovesical junction, and usually present in the same location near the ureterovesical junction (so-called Hutch diverticula). The bladder is seldom trabeculated, and bladder outlet obstruction is not believed to play a significant role.
Acquired diverticula, on the other hand, present in men during the sixth to seventh decade and are often asymptomatic. Various estimates on the incidence in men range from 1% to 8%. Diverticula are typically discovered in men during a workup for lower urinary tract symptoms. Bladder diverticula occur uncommonly in women. When discovered in female patients, they are most commonly attributed to urethral obstruction as a result of bladder neck hypertrophy. This review focuses primarily on diverticula in men; however, the principles and techniques for treating women are the same.
In men, diverticula are believed to be caused primarily by bladder outlet obstruction. This high pressure voiding over time results in a herniation of the bladder mucosa through the smooth muscle layer (muscularis propria). Because of this, diverticula are often thin walled and lack the ability to contract; thus causing stasis of urine, also serving as a nidus for stone formation and infection. Urine stasis has been proposed as a risk factor for tumor formation, and several investigators have reported tumors arising from these diverticula. Diverticula may be single or multiple, and classically appear posteriorly on the bladder near the ureteral orifices, making dissection and excision treacherous. Acquired bladder diverticula have been reported less frequently in modern urologic practice, possibly as a result of advances in the management of bladder outlet obstruction caused by BPH.
BPH and diverticula
Because diverticula are often attributed to bladder outlet obstruction, the role of benign prostatic enlargement (BPE) consequent to BPH must be considered. The natural history of BPH/BPE is progressive, such that trabeculation of the detrusor muscle progresses to the development of cellules, and finally bladder diverticula with deposition of connective tissue throughout the detrusor muscle, ultimately leading to its decompensation if left untreated.
Although causality is commonly implied, there is only circumstantial evidence linking BPH to formation of diverticula. BPH has been noted to be present in 70% of patients with diverticula, compared with bladder neck contracture, which was seen in 12%, and urethral stricture in 12%. Two other important considerations are bladder neck hypertrophy as a primary cause of obstruction and bladder neck contracture following transurethral resection of the prostate (TURP) or radical prostatectomy. Iatrogenic diverticulum formation should also be considered following ureteral reimplantation, and cystotomy and bladder closure. Early work in fetuses has led to the theory that diverticula are located posteriorly at the transition between the thick muscle fibers of the trigone and the thinner fibers of the lateral bladder wall. Other investigators make anecdotal reference to the increased incidence of inguinal and diaphragmatic hernias in adult patients with bladder diverticula, but no data are given to support these observations.
BPH and diverticula
Because diverticula are often attributed to bladder outlet obstruction, the role of benign prostatic enlargement (BPE) consequent to BPH must be considered. The natural history of BPH/BPE is progressive, such that trabeculation of the detrusor muscle progresses to the development of cellules, and finally bladder diverticula with deposition of connective tissue throughout the detrusor muscle, ultimately leading to its decompensation if left untreated.
Although causality is commonly implied, there is only circumstantial evidence linking BPH to formation of diverticula. BPH has been noted to be present in 70% of patients with diverticula, compared with bladder neck contracture, which was seen in 12%, and urethral stricture in 12%. Two other important considerations are bladder neck hypertrophy as a primary cause of obstruction and bladder neck contracture following transurethral resection of the prostate (TURP) or radical prostatectomy. Iatrogenic diverticulum formation should also be considered following ureteral reimplantation, and cystotomy and bladder closure. Early work in fetuses has led to the theory that diverticula are located posteriorly at the transition between the thick muscle fibers of the trigone and the thinner fibers of the lateral bladder wall. Other investigators make anecdotal reference to the increased incidence of inguinal and diaphragmatic hernias in adult patients with bladder diverticula, but no data are given to support these observations.
Concern for malignancy in the diverticulum
Bladder diverticula are known to promote stasis and urinary retention. Some investigations have suggested this increases the risk for malignancy, and the incidence of tumor in a bladder diverticulum has been as high as 10% to 13%. The study by Melekos and colleagues was retrospective, and reviewed a population of men with a mean age of 69 years, all with bladder diverticula, from 1987. This incidence varies widely by author, however, and others have reported incidence of malignancy in individuals with bladder diverticula ranging from 2% to 5.5%. These series seem to include similar patients, all of whom had been diagnosed with bladder diverticula in the fifth or sixth decade, and most are men. Most seem to be from referral hospitals. Because all available studies are retrospective, the possibility of bias must be considered. By comparison, transitional cell carcinoma of the bladder in the general population ranges in incidence from 1% to 2%. Lifetime incidence in North America from Surveillance, Epidemiology and End Results (SEER) data (and referenced by the Urologic Diseases in America project) is 3.5% for men and 1.13% for women. Early reports of tumor arising from diverticula demonstrated poor survival, with 2-year survival ranging from 16% to 58%. One possible explanation for the aggressive behavior of cancer in diverticula is the lack of muscle therein, such that cancer cell penetration into the lamina propria constitutes invasive disease. More recent reports, however, have shown 5-year survival rates of 72%, possibly as a result of earlier detection. Because of the low numbers of patients having both tumors and bladder diverticula, well-powered, prospective, randomized trials do not exist, leading to considerable debate as to how asymptomatic diverticula should be managed when encountered.
Earlier investigators reached a very different conclusion than contemporary investigators. In the largest series to date, Kelalis and colleagues at the Mayo Clinic reviewed their experience from 1955 to 1964 with 285 patients having bladder diverticula. Nineteen of 285 (7%) were noted to harbor malignancy. A subset of 8 patients had been diagnosed with diverticula 1.5 to 16 years before malignancy was diagnosed and were initially treated with outlet-reducing surgery alone. All of those patients subsequently died, and overall, 13 of 19 died within the first year after tumor diagnosis. These patients were treated with partial or radical cystectomy, leading the investigators to “unhesitatingly recommend prophylactic excision” when diverticula are encountered. More recently, Montague and Boltuch described a 90% 2-year survival rate; 62% of these patients were found to have noninvasive, Ta lesions.
Some investigators have now concluded that the presence of a bladder diverticulum poses no greater risk of de novo malignancy and prophylactic bladder diverticulectomy is not warranted. The authors believe that because of the improved prognosis in more contemporary series, prophylactic diverticulectomy is not warranted, but rather periodic surveillance on these patients with voided cytology and cystoscopy is advised. The risk of intervention has to be weighed against the potentially increased risk of missing a malignancy. When high-grade or invasive tumor is found, however, aggressive treatment should be undertaken, including diverticulectomy combined with intravesical chemotherapy or radical cystectomy. Moreover, because there is no muscle or serosa backing the diverticulum harboring a tumor, there is some debate as to whether T1 tumors should be treated differently from conventional transitional cell carcinoma of the bladder. It is clear that great care must be taken during biopsy and transurethral resection (TUR) if cystoscopic management is undertaken, because the thin wall of the diverticulum makes bladder perforation more likely.
Transitional cell carcinoma is the most common histologic type of tumor found in diverticula, with incidence from 80% to 100% of all diverticular tumors, followed by squamous cell carcinoma (7%–15%) and adenocarcinoma (0%–15%). Patients with tumor in the diverticulum most commonly present with hematuria followed by urinary tract infection.
In an effort to further understand the conditions under which malignancy arises from within diverticula, some investigators have examined histologic changes seen in the epithelium of benign diverticula. When Gerridzen and colleagues reviewed their series of 48 cases, they found that only 19% of the diverticula removed demonstrated normal mucosa, whereas 69% demonstrated chronic inflammation, and 13% revealed squamous metaplasia. Kelalis and colleagues noted that 26 of 31 benign diverticulum specimens demonstrated chronic inflammation, cellular infiltration, squamous metaplasia, or leukoplakia. It seems that the mucosa found within diverticula is not normal.
Clinical evaluation
The workup for patients with suspected diverticula shares components with the workup for lower urinary tract symptoms (LUTS), with some important differences. Medical history, American Urological Association (AUA) symptom index score, physical examination including digital rectal examination, urinalysis, and prostate-specific antigen (PSA) (if the patient has a 10-year or more life expectancy) should be undertaken on initial evaluation. Urinary cytology is useful given the possibility of tumor arising from the diverticulum. In addition, serum creatinine is advised for the workup of bladder diverticula, given the chronic nature of the disorder and possibility of concomitant hydronephrosis, which was noted to be present in 6.9% of 115 patients with diverticula from 1 series. Some investigators believe upper tract imaging to assess for hydronephrosis is important, although the AUA BPH guidelines do not recommend this for the workup of BPH. McConnell and colleagues noted in the 1994 Agency for Health Care Policy and Research (AHCPR) meta-analysis that 7.6% of BPH patients had evidence of hydronephrosis, and of those, 33.6% were noted to have renal insufficiency. This was a compilation of 25 investigations including more than 6000 BPH patients evaluated with intravenous pyelogram (IVP) and served as the template for the most recent 2003 AUA Guideline on BPH. The authors perform upper tract imaging on all diverticula because some have been known to cause hydronephrosis. Our study of choice is a computed tomography (CT) scan with contrast, because this gives excellent detail of the bladder and diverticula, and information on the proximity of the distal ureter for posterior diverticula.
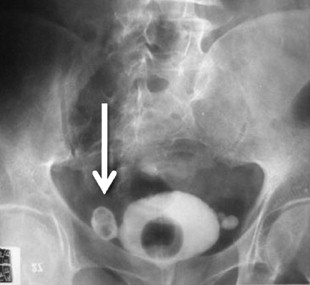
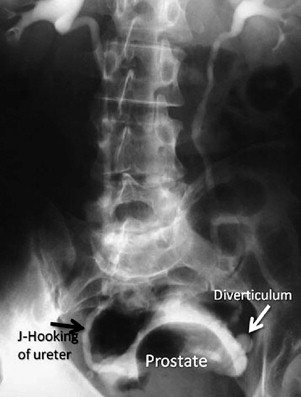
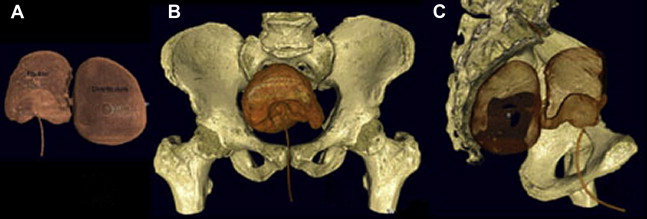
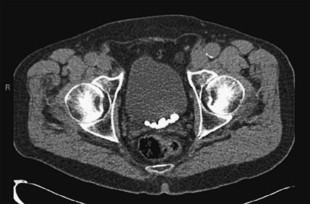
Appropriate imaging classically has included cystogram and IVP. In some instances, this can detect diverticula and filling defects suggestive of a tumor or blood clot ( Fig. 1 ). A filling defect in the area of the bladder neck can denote a large median lobe of the prostate, as shown in Fig. 2 . J-hooking of the ureter is 1 finding suggestive of significant BPH (see Fig. 2 ). CT urogram with three-dimensional reconstruction of the ureters and bladder has replaced cystography and IVP where available ( Fig. 3 ), and conventional CT can be used where three-dimensional reconstruction is not available. CT is more sensitive for detection of bladder and ureteral stones ( Fig. 4 ) than plain radiography. In many cases, CT can be used to measure the size of the ostium leading to the diverticulum ( Fig. 5 A ), or determine the size of a median lobe of the prostate ( Fig. 5 B). CT with delayed excretory phase cuts can help the clinician localize the ureters relative to the diverticulum and thereby aid in the decision on whether to place preoperative stents ( Fig. 6 ). It is important to recognize that diverticula can be multiple. The CT in Fig. 7 demonstrates hydronephrosis bilaterally. This patient presented with renal failure, which improved significantly with intravenous fluid hydration and Foley catheterization before surgery. If CT is unavailable, if there is a contraindication to intravenous contrast, or if ionizing radiation is undesirable to the patient, ultrasound can be considered. It is useful to determine if hydronephrosis is present, but its limitations should be recognized. Ultrasound gives less anatomic detail about the location of the diverticulum relative to the ureters. It is not as sensitive as CT when used to detect ureteral stones although newer techniques have enhanced ultrasonographic imaging of calculi. Magnetic resonance imaging is a useful imaging modality to detect hydronephrosis, but is not as sensitive at detecting stones as CT. Retrograde pyelography is also useful, and can be done at the time of cystoscopy.
Cystoscopy is indicated in all patients with suspected bladder diverticula. It is the most sensitive modality for detecting tumors within the diverticulum. Cytology should also be done. Cystoscopy combines the ability to locate the diverticulum, determine if stone exists in the diverticulum, and gauge the size of the ostium connecting the diverticulum to the bladder. When tumor is suspected within a bladder diverticulum whose lumen cannot be envisioned because of a narrow os, contrast imaging using CT scanning can be helpful as can the use of the flexible ureteroscope.
Voiding cystourethrography (VCUG) is useful for determining if the diverticulum empties when voiding, an important factor in patients suffering from recurrent urinary tract infections. VCUG also detects vesicoureteral reflux. Fig. 8 demonstrates the importance of obtaining lateral views during VCUG, because the diverticulum is not always evident on anterioposterior (AP) views.
Urodynamic studies are helpful in imaging diverticula and determining their association with causative conditions such as bladder outlet obstruction, detrusor overactivitiy, and impaired detrusor contractility. It would be fair to say that videourodynamics is the method of choice when evaluating bladder diverticula. Investigators from Spain have used urodynamic data to compare men with bladder diverticula with similar men with BPH. Men with BPH alone exhibited significant differences when compared with 24 men with bladder diverticula. Postvoid residual was greatly increased (45 mL vs 221 mL, P <.008), and the incidence of urinary retention was also increased in the diverticula cohort (6.1% vs 25%, P <.01), as was the incidence of urinary tract infection (3.1% vs 21.7%, P <.004). Urethral resistance was noted to be much higher in the diverticula group (36.5 cm H 2 O vs 48.5 cm H 2 O, P = .04), and evidence of abdominal straining was also noted more frequently in men with diverticula (23.9% vs 50%, P = .02). Some of these patients received surgical treatment: 11 underwent a bladder outlet procedure alone, whereas 8 received both a bladder outlet procedure and diverticulectomy. The only significant difference between the groups was a decrease in postvoid dribbling in those undergoing diverticulectomy and the bladder outlet procedure (75%) compared with only 10% of the patients undergoing bladder outlet procedure alone. Fig. 9 provides an example of a urodynamic tracing from a man with a diverticulum, elevated voiding pressure, and postvoid residual. High voiding pressures may be more noticeable with a narrow-neck diverticulum than with a wide-mouth diverticulum. The diagnostic steps described earlier are summarized in the algorithm in Fig. 10 for rapid reference.










