Laser-based treatments have emerged in the past 15 years as an alternative to transurethral resection of the prostate (TURP) for treatment of symptomatic benign prostatic hyperplasia. Increasing demand for a minimally invasive procedure to alleviate lower urinary tract symptoms with greater efficacy and fewer side effects has led to the introduction of various lasers. The excellent clinical outcomes, low morbidity, technical simplicity, and cost-effectiveness of GreenLight laser photoselective vaporization have made this technology a valid and efficacious clinical alternative to TURP.
Laser-based treatments have been developed in the past 15 years as an alternative to transurethral resection of the prostate (TURP) for treatment of symptomatic benign prostatic hyperplasia (BPH). Increasing demand for a minimally invasive procedure to alleviate lower urinary tract symptoms (LUTS) with greater efficacy and fewer side effects has led to the introduction of various lasers, including photoselective vaporization of the prostate (PVP) using “GreenLight PV” KTP (potassium-titanyl-phosphate) laser, or most recently, the “GreenLight High Performance System (HPS)” LBO (lithium triborate) laser. Laser prostatectomies may be performed with coagulative lasers such as neodymium:yttrium-aluminium-garnet (Nd:YAG) and diode lasers, cutting lasers such as holmium:YAG, (Ho:YAG) and thulium:YAG (Tm:YAG), or vaporization lasers such as Nd:YAG, Ho:YAG, diode, KTP, and lithium triborate (LBO) lasers. The search for an ideal minimally invasive treatment option for BPH to reduce morbidity and expense is constantly evolving. Many earlier modalities using various delivery systems did not result in consistent and durable outcomes compared with the reference standard, TURP. Since its introduction in 1998 by Malek and colleagues, the excellent clinical outcomes, low morbidity, technical simplicity, and cost-effectiveness of GreenLight laser photoselective vaporization (American Medical Systems, Minnetonka, MN) have made this technology a valid and efficacious clinical alternative to TURP.
Most early data on laser treatment of BPH were based on an Nd:YAG laser using visual laser ablation of the prostate (VLAP), as initially introduced by Costello and colleagues. Limitations included prolonged operative time because of the lack of a continuously emitting laser beam, significant dysuria, and extended postoperative catheterization time secondary to massive sloughing of necrotic tissue. The key determinant of efficacy with laser vaporization is based on the interaction between the laser beam and target tissue. The laser energy (collimated coherent light emitted from an energized source at a single wavelength) can produce either coagulation, when tissue is heated to below the boiling/vaporization temperature but above that required to denature protein, or vaporization, in which the tissue is evaporated by being heated to above the vaporization/boiling temperature. The wavelength of Nd:YAG (1064 nm) is double the wavelength (532 nm) and half of the frequency of KTP or LBO lasers. The KTP and LBO lasers produce the same 532-nm light beam within the visible green region of the electromagnetic spectrum with different maximal average power (80 W vs 120 W, respectively). Unlike the 1064 nm wavelength of the Nd:YAG laser, which is found within the infrared portion of the electromagnetic spectrum, the KTP or LBO lasers are selectively absorbed by hemoglobin within prostatic tissue, thus permitting photoselective vaporization and removal of prostatic tissue by rapid photothermal vaporization of heated intracellular water. With a short optical penetration of 0.8 mm because of the shorter wavelength and absorption by hemoglobin, the resulting coagulation zone is limited to 1 to 2 mm, which leads to a more focused and effective vaporization when compared with the 4 to 7 mm coagulation zone of Nd:YAG. Unlike KTP or LBO lasers, Nd:YAG laser treatment often leads to severe postoperative dysuria and delayed sloughing, resulting in prolonged obstruction.
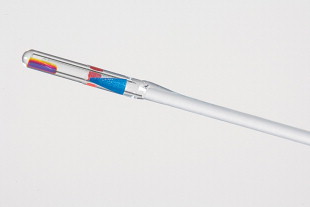
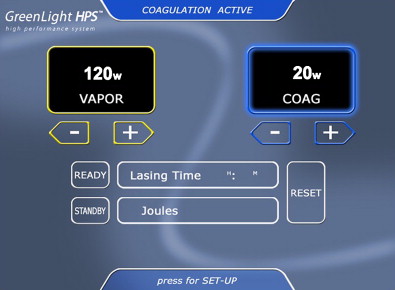
The GreenLight HPS (120-W LBO laser), which is currently used at our institution, permits more rapid and effective tissue vaporization, greater maximum average power, and improved collimation of the laser beam than the older GreenLight PV (KTP laser) system ( Fig. 1 ). For these reasons, the GreenLight HPS laser can be used to treat larger prostate glands with up to 3-mm working distance between the laser fiber and the tissue compared with the required “near contact” of tissue (0.5 mm distance) for vaporization with the older GreenLight PV system ( Fig. 2 ). Increased working distance with the older PV system results in coagulation rather than vaporization. By contrast, the GreenLight HPS uses separate footswitches for vaporization and coagulation (default 20 W) ( Figs. 3 and 4 ). With either system, hemostasis is gained by the inherent superficial coagulative effect of the KTP or LBO laser beam, which permits a nearly bloodless procedure. In the first study of pure KTP, bleeding hemostasis was successfully obtained by defocusing the laser beam (3–4 mm) without the need to switch to Nd:YAG laser for coagulation. Specific studies establishing the efficacy of GreenLight PV and GreenLight HPS laser are discussed later in this review.


Preoperative evaluation may include accurate transrectal ultrasound (TRUS) prostate volume assessment to properly gauge required vaporization energy and operative time. Preoperative evaluation is surgeon-dependent and may include uroflowmetry, postvoid residual (PVR) measurement, cystoscopy, and urodynamic evaluation. In contrast to TURP, no tissue specimen is provided by photovaporization of the prostate (PVP). Therefore histopathologic abnormalities, including high-grade prostatic intraepithelial neoplasia (HGPIN), atypical small cell acinar proliferation (ASAP), and cancer cannot be diagnosed. With preoperative elevated prostate-specific antigen (PSA) or suspicious digital rectal examination (DRE), a TRUS-guided biopsy should be performed. The clinical significance of cancers not identified preoperatively as a result of normal PSA and DRE is not clear. Patients may in the future develop prostate cancer, and should be followed postoperatively by DRE and PSA surveillance in specific circumstances when a proactive follow-up is agreed on by urologist and patient. The procedure may be performed on patients taking 5α-reductase inhibitors for more than 6 months without compromising efficiency or efficacy as described by Araki and colleagues in a prospective nonrandomized trial. GreenLight laser photovaporization has a high absorption affinity for hemoglobin, making prostate tissue a good candidate target tissue. Because 5α-reductase inhibitors reduce angiogenesis and blood vessel formation in prostate tissue, it had been hypothesized that BPH patients treated chronically with 5α-reductase inhibitors would have less significant responses with GreenLight laser photovaporization of the prostate, but this has not been demonstrated. Anesthetic options during the procedure include general and regional anesthesia. Regional anesthesia is effective, but does delay immediate catheter removal. In the outpatient setting, local anesthesia with sedation, oral analgesics, and nonsteroidal anti-inflammatory medications have all been used with excellent safety and recovery profiles, and equivalent outcomes at 2-year follow-up. The efficacy of local anesthetic, periprostatic and pudendal nerve blocks using lidocaine, bupivacaine, or ropivacaine remain controversial. Perioperative antibiotics are given based on urine culture results, and follow-up antibiotics for 3 days are prescribed on an outpatient basis.
Surgical technique
The GreenLight HPS laser procedure begins with careful introduction of a continuous-flow cystoscope with a visual obturator. Blind insertion of the cystoscope without the visual obturator can result in urethral trauma and unnecessary bleeding. Prostate size, prostatic urethral length, ureteral orifice position, and bladder neck are evaluated. If the prostate is too large or friable to inspect without causing bleeding, flexible cystoscopy can be performed initially, although this is rarely necessary. A camera filter insert is placed between the telescope and the videocamera to protect the charge-coupled device chip in the camera from being damaged by the laser light. The laser fiber is inserted into a special laser cystoscope with separation for the fiber and irrigant solution. The authors use a 30 degree cystoscopic lens, as it allows for good visualization of the prostatic urethra and inspection of the ureteral orifices. Alternatively, a 12 degree cystoscopic lens can be used. The KTP or LBO laser generators deliver a continuously emitted beam using a 21 to 23 French continuous-flow cystoscope with a 70-degree side-firing fiber. The continuous nature of the laser beam permits efficient vaporization of gland tissue to the level of the hypovascular prostatic capsule. The routine irrigant is normal saline, although sterile water may also be used without compromising visualization as described by Barber and colleagues in their study of expired breath ethanol measurements during PVP. The goal is to maintain a filled but not over-distended bladder during surgery. Because the electrosurgical resection instruments required for TURP are not used, glycine is not required during photovaporization. This eliminates the possibility of transurethral resection (TUR) syndrome including hypertension, hyponatremia, glycine toxicity, disorientation, and tachycardia, which was traditionally a possible complication during TURP.
The laser fiber has several key markings, including a blue triangle on the quartz cap of the fiber, which is located opposite to where the laser beam fires (see Fig. 2 ). This blue triangle should be visible at all times to avoid damaging the cystoscopic sheath. There is a red stop sign on the opposite side (relative to the blue triangle) of the fiber. This is aligned with the aiming beam of the fiber. The red aiming beam is aligned in the same direction as the laser beam itself and should be directed toward the targeted tissue. The laser beam should not be fired under any circumstance until the aiming beam is visible on the targeted tissue. The laser beam itself exits at a 70-degree forward deflection angle to the fiber axis. The foot pedal has a standby/ready mode pedal in addition to a left vaporization and right coagulation pedal. Default settings on the HPS system include 80 W for vaporization and 20 W for coagulation. The laser can be adjusted accordingly in 10-W increments. The authors routinely start at 60 W and evaluate the effect of the laser on tissue before titrating the power to a higher setting.
The creation of a working space at the start of the procedure is essential; this avoids contact with tissue and consequent fiber degeneration. This working space is formed by enlarging the urethral lumen by strategic removal of prostatic tissue that may limit maneuverability of the laser fiber and cause full contact with the prostatic tissue. The working space facilitates near-contact vaporization and subsequent optimal irrigation and visualization during lasing, defining the bladder neck (proximal limit of vaporization) and the urethral sphincter at the verumontanum (distal limit of vaporization). The tissue is vaporized in a sweeping manner, keeping the fiber in motion at all times at the ideal working distance of 1 to 3 mm from the tissue during vaporization mode. The procedure is started by lasering the median lobe (when present) and the bladder neck. Initiating the procedure at the bladder neck/median lobe ensures sufficient irrigation and visualization during the remainder of the procedure. The lateral lobes are then vaporized in a symmetric manner, with the laser fired selectively against peaks of tissue on the surface, flattening them out rather than targeting areas that have already been vaporized. A smooth surface permits easier control of any bleeding that may occur than if the surface were irregular. Next, lasering of the apex is performed, and this must be precise without lasering distal to the verumontanum to avoid damage to the urethral sphincter. Anterior vaporization may be performed without scope rotation if the fiber is maintained far from the cystoscope. At the end of the procedure, the bladder neck and middle lobe can be further assessed to ensure prior vaporization was complete, because it is sometimes technically simpler to complete the middle lobe after the lateral lobes have been vaporized. If the bladder neck remains elevated after vaporization of the middle lobe, bladder neck incision at 5 and 7 o’clock (or 6 o’clock) may be performed. Tissue should be vaporized down to the prostatic capsule until an unobstructed view of the trigone and a TURP-like defect are obtained. At the completion of the procedure, the bladder should be emptied, bleeders should be identified and coagulated, and the contour should be smooth without significant irregular peaks of tissue.
Throughout the procedure, the laser fiber is moved slowly and constantly with a “paint brush motion” to avoid drilling holes in the prostate tissue; spreading the energy in this way promotes a smooth surface. The rotation speed of the fiber must be adapted to efficiency of vaporization. For example, if vaporization is efficient, rotation speed can be increased, whereas if vaporization is less efficient, rotation speed should be decreased. The rotation angle of the fiber must be limited (eg, from 5 to 7 o’clock) to maintain the angle of incidence of the beam perpendicular to the tissue. Bubble formation is representative of effective vaporization. Direct contact with the tissue should be avoided because excessive heat reflection damages the fiber. If tissue build-up occurs on the fiber at any time, it should be immediately cleaned by 1 of 2 techniques. Gently advancing the fiber against the prostatic tissue and then pulling back the fiber towards the cystoscope may remove the excess tissue most efficiently. This may be repeated several times to remove all tissue. If this is not successful, the laser generator should be put in standby mode, and the laser fiber should be gently removed from the cystoscope and cleaned with a clean sterile towel moving from the tip of the fiber toward the fiber control knob and surgeon. It is critical to maintain a clean fiber to allow for efficient vaporization throughout the procedure and maximal use of the fiber (energy limit is 275,000 J).
Bleeding vessels, although rare, may occur during photovaporization of the prostate. With the HPS laser, the coagulation footswitch is used to cauterize bleeding using 20 W. Power can be increased if needed at the discretion of the surgeon. On the older system without individual foot pedals for vaporization and coagulation, the laser power had to be adjusted on the machine to 20 W, and the tissue distance of 0.5 mm was maintained during coagulation. Alternatively with the older GreenLight PV system, the beam can be defocused by increasing the distance between the fiber and tissue. Bleeding during PVP can be classified as venous, from congested mucosa, or prostate tissue, and can negatively affect visibility. Gentle movement of the cystoscope along the urethral path can reduce such bleeding. Maintaining a sweeping motion with the laser fiber can prevent crater development, which can hinder the ability to obtain hemostasis. The surgeon should be especially careful at the bladder neck, median lobe, and verumontanum, where bleeding is most likely to occur. The authors recommend continuing laser vaporization if bleeding is not severe, as further vaporization of the periurethral tissue can cause bleeding to cease. Arterial bleeding is also possible, and may be noted as pulsatile flow. Smooth rotation of the laser fiber and avoidance of crater formation usually prevents this type of bleeding. An irrigation pump may be used to improve visibility, and coagulation (with reduced power to 20 W) should be obtained by coagulating around the bleed, but not directly on it. With venous or arterial bleeding, the laser should be used to paint circumferentially around the bleeding region. If this is not successful, the Bugbee electrocautery (passed through the laser port) can be used to cauterize the bleeder after irrigation is switched from saline to water. Traditional electrocautery resection can be used if other methods are not effective, although this is rarely necessary.
Gentle movement of the laser fiber and cystoscope are recommended, and, overall, GreenLight laser PVP is a more static approach than the movements used with TURP. One may choose to move the fiber in association with the cystoscope when active, or the cystoscope can remain fixed in position while the fiber is moved. The learning curve for the PVP technique is significantly shorter compared with other modalities such as holmium laser enucleation of the prostate (HoLEP), and outcomes such as blood loss, Q max , PVR, and quality of life were not affected when compared early versus later in the learning curve of a surgeon. Overall, good treatment efficacy occurs early in the learning curve of a surgeon. KTP laser vaporization is also considered to be easier to learn and perform than TURP. Competence occurs following 5 to 20 procedures, with larger glands requiring more training. Nonetheless, a mentorship is essential before performing PVP.
With the GreenLight PV 80-W KTP laser and the GreenLight HPS 120-W laser, patients with normal preoperative urodynamics and no history of urinary retention undergo Foley catheter removal 1 hour postoperatively or 1 hour following resolution of spinal anesthetic in the recovery room, before same-day discharge. Patients who have poor compliance, poor detrusor activity, or elevated PVR on preoperative urodynamics, or history of urinary retention have the Foley catheter removed 2 days following discharge. In a recent study by Spaliviero and colleagues, 70% of patients undergoing GreenLight laser PVP were discharged catheter-free on the day of surgery, and the remaining 30% had their catheter removed on postoperative day 1. Two of 21 patients having the catheter removed on postoperative day 1 required reinsertion 3 weeks post surgery for urinary retention, with successful trial of void 1 day later. Other studies have also shown that catheter removal following the operation or on postoperative day 1 is feasible. All patients at our center are discharged with oral pain medication (oxycodone/acetaminophen), oral stool softener (docusate sodium), oral antibiotic for 3 to 5 days, and urinary tract analgesic (phenazopyridine, hyoscyamine).
The evolution of the GreenLight laser system includes an initial hybrid system, followed by stand-alone GreenLight laser with gradually increasing power as studies proved efficacy and safety. KTP lasers with 532-nm wavelength were initially introduced in BPH treatment as part of a hybrid technique with Nd:YAG laser. KTP laser allowed effective vaporization and excision of prostate tissue, whereas Nd:YAG allowed for excellent coagulation and hemostasis. In the first description of KTP laser prostatectomy, Watson described the 30-W KTP laser used in this hybrid technique, followed by Nd:YAG laser coagulation. The hybrid group (using 30-W KTP laser) was identified to have lower recatheterization rates (33% vs 70.5%) compared with 40-W Nd:YAG alone. During brief follow-up, there was significant postoperative retention and delayed onset of therapeutic benefit. At 2.5-year follow-up, higher power 40-W KTP laser was identified to promote more rapid improvement in symptoms than 20-W KTP laser when either was part of the hybrid technique.
Compared with TURP, the randomized controlled trial by Carter and colleagues demonstrated that a hybrid technique (using 30-W KTP laser) gave similar rates of dysuria and median duration of postoperative catheterization. Results after 1 year showed a higher urethral stricture rate in the TURP arm (10% vs 2%) attributed to the larger scope diameter used in TURP. Postoperative urosepsis was more common in the hybrid group, likely secondary to sloughing necrotic tissue following completion of the procedure. Re-operation rate was similar between the 2 groups (2 patients in the hybrid group vs 1 patient in the TURP group). Improvements in international prostatic symptom score (IPSS), Q max , PVR, and LUTS were similar in both groups at 1.5 years. Another study comparing the hybrid technique to TURP in 100 patients showed equivalent improvements in flow rate and symptoms at 3-year follow-up. Retreatment rates were low with no re-operations required in the TURP arm versus 6% in the hybrid group.
Eventually the hybrid technique, which had prolonged operative time and limited tissue ablation, was replaced by pure KTP laser vaporization as laser power capabilities increased. Successful pure KTP laser vaporization (38 and 60 W) using the canine prostate model and human cadaver model has been described The technique was described as technically easy, safe, and rapid for the hemostatic removal of prostate tissue with a 2-mm-thick rim of coagulation. In 1998, the first clinical trial of pure KTP laser vaporization described 10 patients with prostate glands up to 60 g undergoing successful treatment. Hemostasis was excellent, and catheters were removed less than 24 hours postoperatively with dramatic Q max improvement rate of 142% without recatheterization required. At 3-month follow-up, this study revealed a mean peak flow rate increase of 166%, mean PVR volume decrease of 82%, and mean American Urological Association (AUA) symptom score reduction of 77%. In 2000, 2-year follow-up results of 55 well-selected patients (without history of urinary retention, urethral strictures, previous prostatic surgery, prostate cancer, or neurogenic bladder) showed a statistically significant improvement in postoperative Q max (mean 29.1 mL/s, 278% improvement), PVR (27 mL, 75% improvement), and AUA symptom score (mean 14, 82% improvement). Data from this group showed that recatheterization was still not required, although 4% of patients had postoperative delayed gross hematuria (6–8 weeks) following strenuous physical activity, and 9% of patients had retrograde ejaculation.
Although the first pure KTP laser vaporization studies reported impressive results, the power of the 60-W KTP laser was not adequate to treat prostate glands larger than 60 g in a reasonable time period. To improve the vaporization speed, the GreenLight PV (80-W KTP) laser system was introduced. Total energy delivery is approximately 200,000 J in 1 hour for a prostate of 80 g. The 80-W KTP laser has been shown to be effective with prompt improvement in maximum urinary flow rate and symptom scores in multiple studies with prostate volume reduction ranging between 30% and 44%, and PSA reduction ranging between 30% and 42% ( Table 1 ). Outcomes for the 80-W KTP laser in the study of Hai and colleagues describe reduction in prostate volume of 27% in 10 patients at 1-year follow-up. In several uncontrolled clinical trials, 759 men with prostate weight ranging from 15 to 250 g (mean 49.6 g) were treated in a mean operative time of 53.7 minutes, with reduction in prostate volume of 37% to 53%. No significant bleeding was recorded, and the mean improvement from preoperative Q max was 13.6 mL/s; the mean decrease in IPSS was 14 points. Twelve-month outcome was reported by Te and colleagues from a multicenter prospective study including 145 patients. AUA symptom index (SI), Q max , and PVR were found to be significantly improved as early as 1 month postoperatively. At 12 months, the mean AUA-SI score decreased from 23.9 to 4.3, whereas the mean Q max increased from 7.8 to 22.6 mL/s ( P <.0001). PVR decreased from 114.3 to 24.8 mL and the mean prostate volume was reduced from 54.6 mL to 34.4 mL (37%) ( P <.0001). Twelve-month follow-up was also described in 78 patients from Japan by Seki and colleagues with durable improvement in urinary flow with no effect on serum sodium or hemoglobin levels.









