Historically, benign prostatic hyperplasia (BPH) has been a major focus of urologic practice and surgery. But a simplistic causal relationship among prostatic enlargement, progressive obstruction, lower urinary tract symptoms, retention, and complications of retention has been challenged by recognition of the incomplete overlap of prostatic enlargement with symptoms and obstruction. The result has been a greater focus on symptoms than prostatic enlargement and a shift from surgery to medical treatment. Therefore, the question can be asked whether BPH per se, the glandular enlargement as it contributes to bladder dysfunction, or hyperplastic enlargement as a biomarker for generalized lower urinary tract dysfunction are concerns. This article addresses these issues.
Historically, benign prostatic hyperplasia (BPH) has been a major focus of urologic practice and surgery. However, a simplistic causal relationship among prostatic enlargement, progressive obstruction, lower urinary tract symptoms, retention, and complications of retention has been challenged by recognition of the incomplete overlap of prostatic enlargement with symptoms and obstruction. The result has been a greater focus on symptoms than prostatic enlargement, and a shift from surgery to medical treatment. Therefore, the question can be asked whether BPH per se, the glandular enlargement as it contributes to bladder dysfunction, or hyperplastic enlargement as a biomarker for generalized lower urinary tract dysfunction (LUTD) are concerns. This article addresses these issues.
Recent observational studies have uncovered a remarkable association of BPH with various manifestations of LUTD, including lower urinary tract symptoms (LUTS), erectile dysfunction (ED), and chronic pelvic pain syndrome (CPPS). How does one make sense of this? Should BPH be considered the primary mechanism for LUTD? Or should BPH be considered just one facet of a generalized pathophysiologic process affecting aging men? An unbiased approach would entertain both possibilities: acknowledging the long-recognized role for BPH in the development and progression of LUTS while recognizing that etiologic factors for development of BPH likely have collateral impacts on other facets of lower urinary tract function.
Traditionally, the definition of BPH has been stratified to include histologic BPH, macroscopic glandular enlargement, BPH-related symptoms, and BPH-related complications (retention, renal failure, stones). Although this stratification is appealing in its simplicity, the potentially intertwined nature of prostatic hyperplasia, LUTS, and other symptoms of LUTD suggest that this stratification may not be particularly helpful in sorting out the development and natural history of BPH and associated symptoms.
Etiology
The Etiology of Prostatic Enlargement
BPH is characterized histologically as a progressive enlargement of the prostate gland resulting from a nonmalignant proliferative process that includes both epithelial and stromal elements. Growth results from proliferation of fibroblasts/myofibroblasts and epithelial glandular elements near the urethra in the transition zone of the prostate gland. The hyperplastic process is multifocal and exhibits a variegated histology with variable proportions of stromal nodules and glandular hyperplasia. The histology of BPH was carefully described by McNeal. During the initial phase of BPH small hyperplastic nodules appear in the periurethral area and gradually increase in number. A second phase of BPH, generally occurring in men older than 60 years, involves a dramatic and simultaneous increase in size of glandular nodules McNeal noted that the histologic appearance of stromal tissue in BPH nodules resembled the histologic appearance of developmental mesenchyme and hypothesized that BPH is caused by “embryonic processes reawakened in a distorted form in adult life.”
Endocrine influences have been postulated to play an important role in BPH. Androgen stimulation is required for fetal prostate growth and development but are considered to play only a permissive role in the pathogenesis of BPH. Androgen levels in the prostate are not significantly different in BPH and normal tissues, and currently no evidence shows an increase in BPH incidence for men undergoing androgen supplementation therapy. However, estrogens or a changing ratio of androgens to estrogens in aging men has been speculated to play an important role in the pathogenesis of BPH. This hypothesis is based on two main observations.
First, the ratio of testosterone to estradiol steadily declines in aging men. Second, experimental manipulation of estradiol levels in animal models can cause benign prostatic enlargement. Dogs and humans are believed to be the only mammals with a significant incidence of spontaneous BPH, and treatment of young dogs with androgen plus estrogen hormones leads to an earlier onset and greater extent of benign prostatic enlargement. Similarly, treatment of mice with androgen plus estrogen hormones leads to benign prostatic enlargement (Talo and colleagues 2005; Ishii and colleagues 2006; McPherson and colleagues 2008). Recent studies implicating obesity and BPH could reflect an increased estrogen/testosterone ratio in obese men resulting from increased aromatization of testosterone in peripheral tissues.
Prostatic inflammation is a common feature of the adult prostate and is associated with the development and progression of BPH. Acute and chronic inflammation are extremely common histologic findings in the adult human. McNeal found inflammation in 44% of prostate tissue samples in an autopsy series in men without evidence of other prostate disease, whereas Bennett and colleagues reported inflammation in 73% of prostates examined. The origin of inflammation in the prostate remains a subject of debate and is likely multifactorial. Evidence exists for urinary reflux into the prostatic ducts, and bacterial colonization/infection in surgical specimens of BPH seems to be common. Among patients who underwent transurethral resection of the prostate (TURP) and had preoperatively sterile urine, 38% of specimens grew bacteria when the tissues were morcellated and cultured. Other possible causes of inflammation include noxious dietary constituents, autoimmune mechanisms, oxidative stress associated with androgen action, and systemic inflammation associated with the metabolic syndrome.
A retrospective study of 3942 prostatic biopsies identified as consistent with BPH showed inflammation in 1700 (43.1%; 25). A study of specimens obtained from 80 men who had no symptoms of prostatitis but underwent TURP for treatment of BPH found inflammation to be uniformly present. In another study that evaluated tissue removed with radical prostatectomy, inflammation was found in tissue samples of 35 of 40 patients who had BPH, and prostatic inflammation was associated with significantly greater prostate weight than that observed in patients who had no prostatic inflammation.
In a prospective study of autopsy specimens obtained from 93 men who had histologic evidence of BPH, chronic inflammation was found (primarily in the transitional zone) in 75% of prostates examined compared with 55% of prostates not affected by BPH. Prostate biopsy of 8224 men enrolled in the Reduction by Dutasteride of Prostate Cancer Events (REDUCE) trial showed inflammation in more than three quarters of the biopsies. Chronic inflammation was more common than acute inflammation (78% vs 15%, respectively).
Inflammation also correlates with prostatic enlargement and symptomatic progression. Evidence of inflammation on baseline biopsy in the Medical Therapy of Prostatic Symptoms (MTOPS) trial correlated with prostate volume (41 versus 37 mL; P = .0002), suggesting a significant role in prostatic enlargement. Inflammation also correlated with symptomatic progression, risk for urinary retention, and need for surgery. In a recent analysis of the data from the REDUCE trial, Nickel and colleagues reported a weak but statistically significant association between chronic inflammation and symptom severity.
Several studies have identified associations suggesting metabolic risk factors for the development or progression of BPH. The Baltimore Longitudinal Study of Aging examined whether obesity, fasting plasma glucose, and diabetes were associated with prostatic enlargement. This analysis, authored by a collaborator in this proposal, showed a positive correlation of body mass index with prostate volume. The risk was increased for very obese men. The association of obesity with BPH has been supported by other studies. Hammersten and Hogstedt, 1999 observed that prostatic growth correlated with BMI, and Giaovannucci and colleagues 1994 found that obesity was associated with an increased risk for BPH surgery.
One mechanism through which obesity has been postulated to promote hyperplasia is increased peripheral aromatization of testosterone with a resulting increase in the estrogen/testosterone ratio. Another postulated mechanism involves the association of obesity with inflammation and oxidative stress; factors that have been associated with BPH. Other studies have shown that men diagnosed with BPH have a higher incidence of diabetes than the general population, and that diabetes is associated with more severe symptoms (Michel and colleagues 2000; Hammerstein and colleagues 2001). Part of the explanation may be that diabetes can be a primary cause of lower urinary tract symptoms, but the studies cited earlier suggest that metabolic factors may influence the development and progression of LUTS indirectly by increasing the rate of prostatic enlargement.
Association of Benign Prostatic Hyperplasia and Prostate Cancer
BPH and prostate cancer are dysregulations of prostate growth control that share an increasing prevalence with advancing age. This association was recently reviewed by Alcaraz and colleagues 2009. Furthermore, 83% of prostate cancers develop in prostates where BPH is also present. The zonal location of BPH and cancer is generally distinct, but approximately one quarter of prostate cancers arise in the transition zone. Some reports show that the rate of growth of BPH is correlated with the risk for prostate cancer.
Recent histopathologic studies of human prostatectomy specimens identified lesions characterized by proliferating epithelial cells and activated inflammatory cells (proliferative inflammatory atrophy [PIA]) in juxtaposition to areas of prostate intraepithelial neoplasia (PIN) and prostate carcinoma (CaP). Based on this and subsequent studies, chronic inflammation is now widely considered a critical element in the genesis of CaP, and PIA is now widely considered a likely precursor of PIN and CaP (Palapattu and colleagues 2004). The metabolic syndrome has also been implicated as a risk factor for prostate cancer (reviewed in Alcaraz and colleagues 2009).
In summary, epidemiologic evidence of an association between BPH and prostate cancer is being complemented by discovery of shared etiologic influences that may explain the association.
Etiologic factors for lower urinary tract dysfunction
Recent studies have indentified a tantalizing general coincidence regarding the presence of LUTD, including LUTS, ED, and CPPS, in patients who have BPH. Certainly, LUTS have been historically associated with benign prostatic enlargement. More recently noted is the strong association between ED and LUTS and the finding that both conditions may be improved by medical treatment of either LUTS or ED (Kaplan and colleagues 2006).
The coincidence of pelvic pain in men who have BPH also has been noted recently. Although pain has not classically been considered a feature of BPH, a recent study by Clemens and colleagues suggested considerable overlap of voiding symptoms and pain, with as many of 34% of men who had LUTS reporting pain symptoms. The mechanistic basis for these associations is a subject of considerable interest.
Aging is associated with increasing lower urinary tract symptoms independent of prostatic enlargement. The prevalence of LUTS increases with aging in both men and women ( Fig. 1 ). Comparisons of storage and voiding symptoms show comparable trends of increasing symptoms with age, although the overall prevalence is higher in men. The simplest and probably best explanation for this is that most voiding symptoms are a consequence of nonprostatic factors that operate similarly in both sexes. The higher prevalence in men might be explained by the superimposed effect of prostatic enlargement and obstruction.
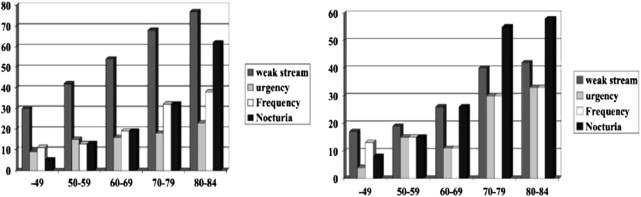
The reasons for the aging-associated increase in LUTS are not well understood. Aging is associated with changes in detrusor morphology, detrusor innervation, and bladder metabolism that may affect bladder function. Partial denervation has been linked to increased excitability of the detrusor, leading to detrusor instability. Aging is associated with decreases in detrusor contractility that result in diminished urinary flow and variable degrees of incomplete emptying. Indirect effects of aging on bladder function may also accrue from degenerative changes in bladder innervation and vascular supply. Coronary artery disease, ischemic heart disease, and vascular risk factors have been found to be associated with BPH symptoms, leading to speculation that pelvic ischemia may be a contributing factor in the development of LUTS.
The recognized association between BPH and ED may reflect a shared etiologic connection with cardiovascular disease. The importance of cardiovascular risk factors in the development of ED is well recognized. Recent evidence suggests that the autonomic and cardiovascular systems may also be involved in the development and progression of BPH and LUTS. Studies have shown a higher prevalence of hypertension in men who have BPH, a positive correlation between the duration of hypertension and prostate size, and a greater risk among men who have hypertension to develop urinary retention and require BPH surgery. These correlations have fueled speculation that hypertension and BPH symptoms are linked by the metabolic syndrome and overactivity of the sympathetic nervous system.
Prostatic inflammation may be a primary cause of lower urinary tract symptoms through influencing bladder sensation and function. Development of prostatic inflammation may trigger and be exaggerated by neurogenic inflammation. Multiple studies support the occurrence of neurogenic inflammation in the bladder, and neurogenic inflammation of the bladder results in many of the symptoms associated with LUTS.
The concept that chronic inflammation accompanying BPH may sensitize afferent nerve fibers of the bladder, resulting in development of LUTS, is supported by the fact that the bladder and prostate share innervation and also the observation that inflammation of one pelvic organ can result in cross-sensitization of other pelvic viscera. Although BPH has not been traditionally considered to be a painful condition and a patient complaint of pain has often been used to distinguish BPH from prostatic inflammation, a far greater number of men diagnosed with BPH describe the presence of pain than was previously recognized. Pain associated with ejaculation has been reported by a substantial number of patients diagnosed with LUTS caused by BPH. In one study of 3700 men who had BPH-related LUTS, 688 (18.6%) reported pain on ejaculation.
The role of prostatic inflammation and afferent sensitization in development of pelvic of pelvic pain is unknown but a promising area of further study.
Epidemiology
The Epidemiology of Prostatic Enlargement
BPH is an age-related process with a histologic prevalence of approximately 10% for men in their 30s, 20% for men in their 40s, 50% to 60% for men in their 60s, and 80% to 90% for men in their 70s and 80s. Androgens and aging are necessary for the development of BPH, but the etiology of prostatic hyperplasia is poorly understood.
Prostate volume increases with age. In the Olmstead study, median prostate volumes were 21, 27, 32 and 34 mL in the 5th, 6th, 7th, and 8th decades, respectively. This study calculated a 1.6% average annual increase in prostatic volume. The rate of enlargement varied considerably at the individual level, but patients who had larger baseline volumes tended to experience more rapid enlargement ( Fig. 2 ). Strong suggestions have been found of geographic variations in prostate size, with several studies showing significantly lower size in Japanese, Chinese, and Indian men compared with American and Australian men (Tsukamotyo and colleagues 1996; Jin and colleagues 1999).
The Incidence of Lower Urinary Tract Symptoms and Urinary Retention in Aging Men
LUTS are prevalent among aging men (see Fig. 1 ). Surveys of an unselected population of men aged 40 to 79 years in Olmstead county, Minnesota, showed moderate to severe symptoms in 13% of the men aged 40 to 49 years and 28% of men older than 70 years. Symptoms of urgency, nocturia, weak stream, intermittency, and sensation of incomplete emptying were most strongly correlated with age (Chute, 1993). Bosch and colleagues surveyed 502 men aged 55 to 74 years in the Netherlands using the International Prostate Symptom Score (IPSS) and identified a prevalence of severe and moderate symptoms in 6% and 24%, respectively.
In a comprehensive review of the epidemiology of acute urinary retention that combined data from various epidemiologic studies, Roehrborn found that the estimated incidence of acute urinary retention is 0.5% to 2.5% per year.
What is the Relationship Between Benign Prostatic Hyperplasia and Lower Urinary Tract Symptoms?
Recent studies have highlighted the comparable incidence of LUTS in men and women, and implicated various potentially contributing factors, including the effect of aging on the bladder and nervous system, and the effects of metabolic derangements, autonomic overactivity, diabetes, neurologic disease, and age- and cardiac-related changes in the pattern of body-water regulation. What does this signify regarding the role of BPH and prostatic enlargement in the development of LUTS?
The Olmstead County Study of Urinary Symptoms and Health Status showed that prostatic enlargement, peak flow rate, and LUTS were all age-dependent. Analysis of the data adjusting for age showed that men who had significant prostatic enlargement (>50 cm 3 ) were 3.5 times more likely to have moderate-to-severe LUTS ( Fig. 3 ). This finding suggests that significant prostatic enlargement is a significant driver in development of LUTS. However, the overall contribution of prostatic enlargement to LUTS in this unselected group of men was calculated to be small. Similarly, Bosch and colleagues observed only a weak correlation of prostate volume with IPSS, peak flow, and post-void residual urine volumes.
The explanation for this is simply that most men, even those who have significant symptoms, have prostate volumes less than 50 mL. In an analysis of 354 symptomatic men, Vesely and colleagues reported a mean prostatic volume of 40.1 ± 23.9 cm 3 . Ezz and colleagues reported a mean prostate volume of 43 ± 20 cm 3 among 803 patients who had mild to severe LUTS. One might infer from these data that prostate volume is an important determinant of symptoms in men who have significant prostatic enlargement but not in those who have only modest degrees of enlargement.
An interesting study examining the correlation of specific parameters of prostate enlargement to symptoms found that length of the transition zone was the dimension most strongly correlated with symptom severity. This study suggests that prostatic enlargement alone does not determine symptom severity, but also the elongation of the transition zone and, presumably, its effect on outlet resistance.
What is the Relationship Between Obstruction and Lower Urinary Tract Symptoms?
Several different studies have shown a significant correlation between diminished urinary flow rate and LUTS (see Fig. 3 ). However, maximum flow rate is a function of both detrusor function and outlet resistance. The correlation of maximum flow rate therefore may reflect a contribution of impaired detrusor function, increased outlet resistance, or both. Nitti and colleagues performed urodynamic studies on 83 patients (mean age, 67 years) who had symptoms of BPH. Of these, 34% were considered obstructed, 20% deemed unobstructed, and 46% believed to be equivocal according to the Abrams-Griffiths nomogram. No significant differences were seen in total, obstructive, or irritative scores among the three groups. In fact, the urodynamic parameter that exhibited a significant correlation was detrusor instability, present in 54% of patients who had irritative symptoms.
Netto and colleagues performed urodynamic studies on 217 patients who had moderate or severe symptoms and identified obstruction in 53% and 83%, respectively. Yalla and colleagues performed urodynamic studies on 125 men (mean age, 67.7 years) who had micturitional urethral pressure profilometry (MUPP) and observed a prevalence of obstruction in 76% and 78% of men who had moderate or severe symptoms, respectively. No correlation was observed between the severity of obstruction and the severity of symptoms. In another urodynamic study of 222 patients who had a clinical diagnosis of BPH and a maximum flow rate of less than 15 mL/s, 80% were obstructed.
Finally, the ICS-“BPH” study evaluated 933 patients who had LUTS. Of this group 57% were obstructed. The presence of obstruction was significantly correlated with urgency and urge incontinence but not with any other symptom. Although these studies show that urodynamic evidence of outlet obstruction is prevalent in men who have moderate to severe LUTS, with an incidence ranging from 34% to 80%, the presence or absence of obstruction does not reliably correlate with either specific symptoms or their overall severity.
Given the similar incidence of LUTS in men and women and the incomplete association of urodynamic evidence of obstruction with symptoms in men, it is tempting to deemphasize the role of outlet obstruction. However, aohHoqwlthough other etiologic factors are important, perhaps even more so than outlet obstruction, the preponderance of evidence indicates that outlet obstruction is present in most men who have moderate to severe LUTS. Furthermore, surgical treatment of outlet obstruction remains a highly effective therapy for LUTS, with symptomatic success rates that clearly outshine any other medical therapy.
If the pathophysiology of LUTS is multifactorial and prostatic enlargement and obstruction is a major contributor in only some patients, why does surgery work so well in most patients?
In an editorial comment on the paper by Yalla and colleagues John McConnell pointed out that urodynamic evidence of obstruction is not a prerequisite for surgery. He noted that the success rate for surgery is higher for patients who have obstruction, but pointed out that most patients who do not have obstruction who undergo transurethral resection of the prostate also experience successful symptomatic outcomes. Nothing in the field of BPH-related research has been as perplexing as this simple fact: that surgical interventions for outlet obstruction improve symptoms remarkably well, even in patients for whom urodynamic testing does not show obstruction. One potential explanation for this paradox is that nomograms for diagnosing obstruction are unreliable, particularly in those who have impaired detrusor contractility. These patients may well be obstructed and would benefit from surgical reduction of urethral resistance. Thus, there is room for healthy debate about the mechanism through which surgery produces symptomatic improvement and whether it depends on reducing outlet resistance.
An intriguing and instructive parallel may be found in the use of afterload reduction in treatment of congestive heart failure (CHF). The heart experiences several age-related changes. These include myocyte apoptosis and myocyte hypertrophy, impaired mitochondrial respiratory enzyme function, a shift in myosin isoform from rapid to slow ATP hydrolyzing forms, increased extracellular collagen and elastin, diminished compliance, prolonged ventricular contraction and slower left ventricular filling, decline in the number of sinoatrial pacemaker cells, increased atrioventricular delay, and increased ectopy. Aging has been termed blunted hypertension , and hypertension has been considered accelerated aging . This symmetry reflects a common mechanism in the effects of aging and hypertension on the heart: both result in increased energy use, increased glycolysis and reactive oxygen species generation, decreased antioxidant defenses, and increased free-radical damage. Oxidative stress is considered the major mechanism for aging and stress-related damage and seems to be a final common pathway for the synergistic effects of aging and hypertension on the heart.
When of sufficient severity, damage to the heart produces the condition of heart failure. Afterload reduction is a central therapeutic intervention for CHF. Reduction of the heart’s workload globally improves cardiac function and ameliorates the symptoms of heart failure. What is noteworthy and particularly important is that afterload reduction is an effective treatment of CHF, even when increased afterload (ie, hypertension) is not a contributing factor. In other words, reducing the afterload globally improves heart function even when the afterload is not significantly increased.
The aging bladder has many similarities with the aging heart. Clinically, diminished bladder capacity, diminished compliance, detrusor overactivity, decreased contractility, decreased Q max and incomplete emptying are seen. The physiologic and cellular changes associated with aging include myocyte hypertrophy, increased electrical coupling, increased ectopic activity, impaired mechanical coupling, impaired mitochondrial enzyme function, decreased contractility, increased extracellular collagen/elastin deposition, and diminished compliance (Elbadawi and colleagues 1998). Remarkably, animal models of bladder outlet obstruction produce many of the same changes, including myocyte hypertrophy, impaired mitochondrial enzyme function, decreased contractility, increased extracellular collagen/elastin deposition, diminished compliance, increased work demand, and increased energy use.
As in the heart, the deleterious effects of aging and increased afterload on the bladder share a common mechanism, including increased glycolysis and free radical generation, decreased (mitochondrial) antioxidant defenses, and increased oxidative stress and free-radical damage. This comparison suggests that the effects of aging and outlet resistance synergize to increase exposure and susceptibility to free radical damage to the detrusor, and that LUTS are a symptomatic manifestation of these degenerative changes in the same way that symptoms of CHF are for the heart. Recalling the central role of afterload reduction in the treatment of CHF, TURP may work in the case of LUTS not by relieving obstruction, per se, but by reducing afterload. This conceptualization coincides with the empiric observation that TURP is highly effective in treating LUTS even when obstruction cannot be shown through urodynamic criteria. As long as the peak flow rate is less than 15 mL/s, wherein some combination of increased outlet resistance or detrusor contractile dysfunction may be inferred, surgery to reduce outlet resistance is highly successful.
This analysis accounts for the multifactorial origin of LUTS in aging men and women, recognizing a special role for prostatic enlargement in some men, and identifies TURP as an intervention that is uniquely effective in men because surgical reduction of afterload is usually only applied in men. Anecdotal success has been observed in treating women who have bladder neck obstruction and LUTS with TURP.
With respect to the understanding of BPH/LUTS and the role of surgery, this suggests that past attempts to understand and predict the efficacy of surgery for LUTS based on obstruction have failed not only because the definition of obstruction is relative and arbitrary but also because the efficacy of surgery lies in reducing outlet resistance and decreasing afterload rather than remedying a pathologic condition of abnormally increased outlet resistance.
Epidemiology
The Epidemiology of Prostatic Enlargement
BPH is an age-related process with a histologic prevalence of approximately 10% for men in their 30s, 20% for men in their 40s, 50% to 60% for men in their 60s, and 80% to 90% for men in their 70s and 80s. Androgens and aging are necessary for the development of BPH, but the etiology of prostatic hyperplasia is poorly understood.
Prostate volume increases with age. In the Olmstead study, median prostate volumes were 21, 27, 32 and 34 mL in the 5th, 6th, 7th, and 8th decades, respectively. This study calculated a 1.6% average annual increase in prostatic volume. The rate of enlargement varied considerably at the individual level, but patients who had larger baseline volumes tended to experience more rapid enlargement ( Fig. 2 ). Strong suggestions have been found of geographic variations in prostate size, with several studies showing significantly lower size in Japanese, Chinese, and Indian men compared with American and Australian men (Tsukamotyo and colleagues 1996; Jin and colleagues 1999).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







