The high-powered holmium laser is an excellent tool for the surgical treatment of benign prostatic hyperplasia. This article discusses the background of holmium use in the prostate and describes the surgical techniques of holmium laser ablation of the prostate and holmium laser enucleation of the prostate. Operative challenges are reviewed with suggestions as to how to avoid these problems or deal with them when they arise. Surgical outcomes and a thorough literature review are both presented.
Over the past decade, urologists have witnessed an expansion in the number of various techniques used for the treatment of benign prostatic hyperplasia, especially in the arena of laser surgery. The use of neodymium: yttrium aluminium garnet (Nd:YAG) laser technology in treating benign prostatic hyperplasia (BPH) was initially described in 1992 by Costello and colleagues, representing the first published description of laser prostatectomy.
Shortly thereafter, Gilling and colleagues described the use of holmium: YAG (Ho:YAG) in the ablation of prostate tissue, and although holmium laser technology had well-established applications in treating urinary calculi, this was its first application in treating the prostate. They developed a combination approach using holmium to create a channel in the prostate and the Nd:YAG to coagulate the prostate (holmium laser ablation of the prostate, or HoLAP). However, they discovered that holmium could be used alone and had fewer side effects, but the process was slow and tedious with the 60 W laser unit currently available.
This group then expanded on the technique by combining holmium laser resection of the prostate (HoLRP) with mechanical morcellation (holmium laser enucleation of the prostate, or HoLEP). Since then, a plethora of studies have been published touting the procedure and it has slowly gained popularity, particularly outside the United States. Outcomes have been as good as traditional methods, with recently published 10-year data showing sustained results over time. HoLEP has distinct advantages over other surgical approaches, including efficacy despite prostate size, low morbidity, and shorter hospitalizations. The American Urological Association’s guidelines on the treatment of BPH include HoLAP and HoLEP, and dedicated CPT codes exist for each procedure. This article describes the holmium wavelength and its benefits in prostate surgery, discuss indications of surgery, describes the surgical technique of both the modern HoLAP and HoLEP and suggested postoperative care, and reviews published studies supporting both techniques as excellent treatments for men experiencing bladder outlet obstruction secondary to the prostate.
Physics
Both Ho:YAG and Nd:YAG are crystals used as active laser media in solid-state lasers. Solid-state lasers use a solid rather than liquid or gas medium to derive optical gain within the laser. The benefits of Ho:YAG over Nd:YAG laser are partly related to their differences in wavelength. The holmium wavelength (2140 nm, nonvisible/infrared) is absorbed by water. The depth of penetration is 0.5 mm, and if the laser is more than that distance from the target (ie, prostate), it has no effect on tissue and the energy is dissipated in the water. Nd:YAG has a shorter wavelength (1064 nm), resulting in deeper tissue penetration and more thermal injury.
Ho:YAg has the additional advantage of being a pulsed solid-state laser, which leads to a shorter absorption length. Both holmium and YAG have excellent hemostatic properties and can be used in normal saline, negating issues related to hyponatremia and operative time, which are described later.
The drawbacks of the Nd:YAG are that the tissue effects can cause edema, heating of the irrigant fluid, and deeper tissue injury, leading to prolonged catheter time, delayed clinical improvement, and irritative symptoms that can be severe and persistent. Therefore, YAG has fallen out of favor and lasers such as holmium and potassium titanyl phosphate (KTP) have become the preferred wavelengths.
Holmium energy is delivered through small, flexible fibers and is controlled with precision by the operating surgeon. Energy is stored within the laser resonator and released in a pulsed fashion, controlled with a foot pedal. Energy travels along the laser fiber through internal reflection that bounces the energy down the fiber. Specialized clear lens protective eyewear is recommended for the operating physician and those working with the fiber. For the patient, having the eyes closed or blocked by a sheet is sufficient. The machine is key-controlled and easy to use. Regular maintenance is recommended to ensure proper functioning, and blast shield replacements should be kept in stock.
Hemostasis
The hemostatic effects of holmium are believed to be related to the physics of the laser energy. How the laser energy interacts with the tissue reflects primarily the wavelength, time of energy application, and the energy density (fluence). The wavelength and interaction with the target tissue determines the efficiency with which the energy is transferred to the tissue. The holmium wavelength has a high absorption in water.
For the prostate, which is a tissue type that has a high density of water, the wavelength has a very favorable absorption coefficient, implying good tissue conductance of thermal energy. The energy is absorbed by water in the prostate cells and the tissue is heated to 100°C, effectively causing vaporization/ablation. Coagulation occurs if the tissue is heated to temperatures greater than 70°C but less than 100°C.
Holmium energy is delivered through a fiber that can be controlled to produce either ablation or coagulation. By lowering the fluence, either through decreasing the energy pulse or pulling the fiber tip away from the tissue, the tissue temperature is lowered and ablation does not occur; rather, the heat is absorbed into the tissue, resulting in coagulation. These properties come into effect when treating the prostate, regardless of whether the patient is on anticoagulant medication. The water content of the cells is no different, and therefore the hemostatic properties are the same. Given its hemostatic properties, no limitations exist to its use on patients taking blood thinners. Patients are sent home the same day of surgery or the following morning after an overnight observation.
Surgical Indications
Any patient who has voiding symptoms secondary to bladder outlet obstruction is a candidate for holmium surgery. Patients who have glands less than 60 g can be considered for HoLAP or HoLEP. However, gland sizes greater than 60 g are best suited for HoLEP, because ablation would be time-consuming. Basically, patients who urologists would consider good candidates for transurethral resection of the prostate (TURP) or open prostatectomy can undergo holmium surgery.
In addition, patients on anticoagulation medication are treated effectively with holmium, even when taking therapeutic levels of coumadin or platelet aggregation inhibitors, such as clopidogrel bisulfate or aspirin. Overall bleeding, regardless of coagulation status at surgery, is much reduced in all patients compared with that seen during transurethral resection (TUR) and open procedures. Because normal saline is used during resection, TUR syndrome risk is eliminated. Fluid absorption is minimal given coagulation of the blood vessels with tissue resection. These decreases in risk for operative effects may allow more fragile elderly patients to undergo definitive therapy for which they might otherwise not be deemed medically fit.
Instruments and Settings
HoLEP is an endoscopic approach that follows principles similar to those of open prostatectomy. In essence, the laser fiber becomes the finger that is used in open surgery. A standard setup includes a 26F resectoscope with laser bridge, 30° lens, 5F ureteral catheter, 80 or 100 W holmium laser unit, and a 550-μm end-fire laser fiber. Olympus and Storz have resectoscope adaptors that can be used, whereas other companies such as Wolf have dedicated laser resectoscopes.
The laser is set at 2 J and 40 or 50 Hz, depending on the total wattage of the laser unit. For tissue retrieval, necessary equipment includes a morcellator, morcellator tubing, tissue retrieval sock, retrieval loop, long nephroscope lens, and percutaneous nephrolithotomy grasping instruments.
Various practitioners have used slightly modified equipment choices, including a 28F resectoscope, 7F ureteral catheter, and an offset lens for use with morcellation that can be placed within the same resectoscope sheath. Use of the lens resectoscope adaptor (as is the authors’ preference), negates the need to exchange the resectoscope for a nephroscope during morcellation.
For HoLAP, the equipment used is similar, except that no morcellation/tissue retrieval instruments are necessary. The 550-μm side-fire fiber is used. This fiber is surrounded by a casing that makes the outside diameter larger than the end-fire fiber, and therefore no ureteral catheter is necessary if a laser bridge is used. The most common settings are those used for HoLEP at 2 J and 40/50 Hz. Although other settings have been described, the most recent trend has been to use these.
Surgical Technique: Holmium Laser Enucleation of the Prostate
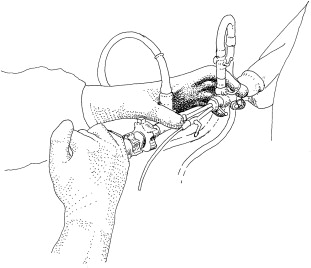
The urethra is dilated with sounds, if indicated, and a resectoscope is placed. The laser fiber is passed through the ureteral catheter, which is then advanced through the laser bridge. The ureteral catheter helps stabilize the fiber and prevent bouncing. The catheter should be extended no more than a millimeter or two from the end of the scope to prevent obstruction of view. The laser fiber should be out beyond the edge of the catheter, but kept in fairly close control. The hands are positioned on the scope as shown in Fig. 1 , which allows for better movement and rotation of the scope during the procedure.
Incisions are made in the 5 and 7 o’clock positions to the level of the surgical capsule, which is easily identified by the presence of parallel fibers, a distinct difference from the “fluffy” appearance of the prostate. Incisions are extended from the bladder neck to the verumontanum. A sweeping motion is used to create a wide groove. Once the two grooves have been created, the fiber is used to enucleate the middle lobe along the surgical capsule in a retrograde fashion from the veru toward the bladder neck. The beak of the scope can be used to peel the adenoma along the capsule, toward the bladder neck, by placing the beak underneath the lobe and pushing upwards. Those on 5α-reductase inhibitors may not peel well, because the capsule in these men is much more adherent to the adenoma.
Bleeding can be controlled by defocusing the fiber over the bleeding site and holding the fiber in place for several seconds. The settings can be changed to 2.5 to 3 J and 35 to 40 W for coagulation, if desired. If the bleeding is recalcitrant, treating the tissue around the site may be successful.
Once the middle lobe is resected, attention is directed to the lateral lobes. The urinary sphincter is identified and a groove is made along the capsule at the level of the veru beneath the apex of the prostate and continued laterally and anteriorly. The scope is used to negotiate the turn, rotating and pushing the scope along the capsule. The fiber is kept constant and fixed and is used to separate the adenoma from the capsule. Again, a peeling motion can be used, with the scope lifting and pushing the gland off the capsule toward the bladder neck. This technique will likely not be successful in patients on 5α-reductase inhibitors. With small prostates, this lateral motion can be continued up along the anterior surface and across the midline to the opposite side.
For larger prostates, the scope is turned to the 12 o’clock position and a midline incision made in the prostate from the bladder neck to the level of the veru, again down to the capsule. The incision is widened with the same sweeping back and forth motion, creating a trough. The incisions are then connected from the lateral edge to 12 o’clock and retrograde enucleation is performed.
Morcellation
Once all three lobes have been enucleated and passed into the bladder, the laser bridge is exchanged for the morcellator bridge. If no morcellator bridge is available, the resectoscope is exchanged for a rigid nephroscope. Normal saline irrigation is essential in this portion of the procedure, and the bladder must be kept full. The morcellator is passed through the working channel of the offset lens, and the prostate pieces engaged. Depression of the pedal halfway initiates the suction component of the morcellator. Once the lobe is engaged, the morcellator and scope are pulled back to the bladder neck and directed up off the bladder floor. Positioning the morcellator at the bladder neck, which is more fixed, helps avoid inadvertent engagement of the bladder wall, particularly with large, floppy bladders. The pedal is depressed fully and the reciprocating blades morcellate and suction the tissue through the device, which is collected in a retrieval sock and sent to pathology. Pieces that are small or cannot be morcellated can be collected through the retrieval loop or stone grasping instruments.
Surgical Technique: Holmium Laser Ablation of the Prostate
A laser bridge is recommended for ablation because it keeps the fiber in a predictable location and prevents bouncing. The 550-μm side-fire fiber is advanced through the bridge and out through the end of the scope until the solid line is visible. This line must been seen throughout ablation to avoid injury to the resectoscope and lens. The aiming beam must be identified (70° angle from the fiber) and tissue ablation begun, keeping the fiber close to, but not touching, the prostate. The fiber should be rotated slowly, with the surgeon avoiding going too fast, which is a common mistake. The surgeon must allow sufficient time for the energy to be absorbed; rapid rotations only heat the irrigant and do not ablate tissue.
Surgeons tend to develop an approach that works well for them, with the goal being to ablate all adenoma until prostatic capsule is visualized circumferentially from the bladder neck to the veru. The authors start with troughs at 5 and 7 o’clock, ablating the middle lobe to capsule, then proceeding to the lateral lobes. Starting at the bladder neck, adenoma is ablated to capsule, using the handpiece to rotate the fiber over the surface of the lateral lobe from the middle lobes troughs to 12 o’clock, gradually moving distally toward the veru. Once capsule is visible throughout, the contralateral lobe is addressed.
Postoperative Care
Whether HoLEP or HoLAP was performed, the authors leave a catheter in place overnight, generally a 20F standard two-way catheter. Overstretching of the bladder during the procedure may cause transient detrusor dysfunction, and an overnight catheter reduces the risk for a return visit to the emergency department. Other urologists remove the catheter the same day, but generally only after HoLAP. If a patient was performing self-catheterization or was in urinary retention preprocedure, experts recommend leaving the catheter in place for 3 to 5 days and having it removed at home by the patient or in clinic after a voiding trial. Most patients are discharged the same day as the procedure or the following morning after an overnight observation. Patients who are therapeutically anticoagulated may be kept overnight, but rarely is three-way irrigation required. Patients are seen at 4 weeks for measurement of uroflow and post-void residual. Further follow-up after that point is case-specific.
Bladder Recovery
Depending on the stage of bladder decompensation before surgery, some recovery process of the bladder is expected. Like those that would be encountered after a TURP or open prostatectomy, the symptoms include short-term exacerbation of irritative voiding symptoms, transient incontinence (stress and/or urge), retrograde ejaculation, urethral strictures, and urinary retention. Dysuria is uncommon with holmium laser treatments.
The stress symptoms are similar to those encountered after an open prostatectomy, given the volume of tissue removed with HoLEP, which is greater than that for TURP and ablative techniques. The prostatic fossa is often large postprocedure, and can lead to urine trapping and leakage with stress maneuvers in the short-term. In addition, the resectoscope is positioned across the urinary sphincter for a longer duration than in TURP and the authors believe transient sphincter dysfunction can occur. However, as with TURP and open procedures, most patients resolve their voiding issues with time and bladder recovery. Anticholinergic medication should be used in patients who have urge symptoms. The duration of recovery is individual and this should be stressed to patients before surgery.
Holmium laser enucleation of the prostate: tricks, tips, and tribulations
Don’t Start with Mt. Everest!
The most important tip is to start with prostates no larger than 60 g until the technique has been mastered. In fact, for the first 10 cases, glands smaller than 40 g are ideal. Once the technique is understood, then larger glands can be worked on and many potential problems can be avoided altogether, or easily addressed. An option to trilobar enucleation is to begin with 5 and 7 o’clock incisions and middle lobe enucleation with an end-fire fiber, followed by lateral lobe ablation with a side-fire fiber. Once the urologist is comfortable with the incisions and middle lobe, the surgeon can progress to the lateral lobes.
Undermining of the Bladder Neck
With standard TURP, because the loop works from the top down, surgeons have a clear view of the bladder neck during resection. With HoLEP, by staying on the capsule and working in a retrograde fashion, the bladder neck can be undermined. Most of the time, this is not dangerous or even problematic, but can lead to fluid extravasation around the bladder in the pelvis. This issue can be easily avoided by identifying the lobes at risk for undermining and thereby modifying the technique. Lobes at risk are those with a sharp upwards angle into the bladder (high bladder neck), requiring that the lobe be lifted extensively. It can be challenging to direct the fiber upwards under the lobe. In cases like this, the surgeon should either leave a rim of prostate tissue and make a more superficial incision plane during enucleation to reduce the angle, or remove the lobe working from lateral to medial in a V formation. Once the most distal portion of the lateral lobe is free, the lobe will flip upwards and the degree of the angle will be reduced, making it easy to resume the back and forth sweeping motion with the fiber.
Middle Lobe that Extends into the Bladder and Splays the Trigone
Provided that the surgeon stays on capsule, no injury should occur to the ureteral orifices. The resection may be close to the orifices, but injury is unlikely. Even if mucosa near or surrounding the orifices is ablated, penetration of the holmium energy is so superficial that injury, stricture, or obstruction is low risk. To the authors knowledge, no significant ureteral injuries have occurred with HoLEP.
Middle Lobe Just Proximal to Veru
When making incisions at the 5 and 7 o’clock positions, the portion of the gland just proximal to the veru can be large and posterior. The scope often must be towed down and a straight incision made to open up the gland. Once this cut has been made, the resectoscope can be used to retract the lobes and then the incision continued to the capsule.
Anterior 12 O’Clock Position
Most urologists early in the learning phase find this incision to be challenging. Often, the incision is not carried distal enough because of concern for injuring the sphincter. If the incision is too short, the lobe will not advance toward the bladder, making the retrograde enucleation difficult, or two parallel incisions will be made. Familiarity with the procedure makes this incision easier to perform.
Urethral Mucosa at Anterior Distal Edge
If the anterior incision is not made distal enough, or the lateral incision not extended enough anteriorly, urethral mucosa at the anterior position over the veru will prevent advancement. This occurrence is best identified by pulling the scope back to the veru and looking up to 12 o’clock; it is often easy to see the mucosa pulling and an incision can be made under direct visualization. Once the mucosa is incised, the planes become obvious and the remainder of the case is generally straightforward.
Creation of Two Incisions
Surgeons must make sure the lateral and anterior incisions match. Creating two parallel incisions can create confusion, disorientation, and inefficiency. If the surgeon stays on capsule throughout, this is avoided. If two incisions are made, the surgeon must get orientation, find the bridge between the two incisions, incise the bridge, and get back on track. Starting with small prostates will help with this portion of the learning curve.
Difficulty with Suction
Unlike TURP where the scope is off the prostate bed, with HoLEP the scope is towed down. Because the suction ports are located underneath the resectoscope, times may occur during the procedure when the undersurface of the scope is in contact with the prostate and suctioning of fluid does not occur. Removing the suction tubing and allowing for gravity drainage is the best course of action. Once the middle lobe is removed, this generally does not occur because the channel is then more open.
Morcellation: Poor Visualization from Bleeding
Poor visualization caused by bleeding is rare, but can be frustrating. Depressing the pedal of the morcellator part way to flush the bladder often helps, as does compression of the prostatic fossa with the scope. The bladder can also be filled to capacity, which may compress any bleeding vessels. Once the piece is engaged in the morcellator, visualization is generally good. If visualization remains poor, the pieces can be left and the patient brought back to the operating room in a few weeks. The lobes are large, will float around in the bladder, and are unlikely to obstruct, and therefore no Foley is required. Morcellation of these retained lobes is often easy and quick.
Engagement of Bladder Mucosa with the Morcellator
The mucosa of the prostate lobes is present on the resected lobes and, when morcellating, differentiating between bladder mucosa and prostatic urethral mucosa can sometimes be worrisome. These mucosa can be defined by engaging the lobe and then, before morcellation, turning and rotating the lobe, which clearly shows the resected edges. If the bladder mucosa is inadvertently engaged, the suction tubing should be removed from the morcellator and the device gently pulled free of the mucosa. Any bleeding is often minimal and can be left alone or treated with the laser. Bladder injury can be easily avoided if strict rules are followed. One should avoid “chasing pieces” and allow the lobe to come to the morcellator, ensure the bladder is full, and position the morcellator off the bladder floor back near the bladder neck.
Morcellator Does not Seem to be Morcellating
Depending on the composition of the prostatic tissue, some lobes morcellate more quickly than others. If the pieces are not morcellating, the tissue may need to be reengaged. With the pedal depressed, the morcellator can be pulled gently in and out of the scope, which frees the lobe and then reengages it in a different location. If the tissue is clearly not cutting, it may be time to change the blades. Each set of blades is good for approximately 8 to 10 cases.
Inability to Engage Pieces into the Morcellator
Small round pieces may not seed into the morcellator blades. If the piece has a rough edge, the lobe should be engaged at this location. If it is round in all directions, the piece should be retrieved with the retrieval loop or a stone grasper.
Inability to Remove Pieces Beyond the Sphincter
When using the retrieval loop or stone graspers, the piece must be kept close to the scope and the scope used to keep the sphincter open. The normal curve of the urethra must be followed rather than trying to pull the piece straight out. Once the piece is distal to the sphincter, a finger can be placed in the perineum to help advance the piece through the urethra. Very large pieces can be removed in this fashion.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








