- Ascites in a cirrhotic due to liver failure is a bad prognostic sign. MELD (see Chapters 2 and 4) should be calculated and liver transplantation should be considered. Low albumin states can precipitate ascites in the setting of cirrhosis without liver failure—the MELD is low and so liver transplantation is not the answer.
- Worsening ascites should prompt a search for a cause precipitating the clinical deterioration, such as bacterial infection or renal failure.
- Treating the underlying liver disease to prevent further liver injury can result in resolution of ascites and preventing the need for liver transplantation.
Introduction
It has long been recognized that the development of cirrhotic ascites is associated with a high mortality risk.1 D’Amico et al. have reported that, in a population where the underlying liver disease was largely untreated, the development of ascites is associated with a 20–26% chance of death over the next 1 year.2,3 Many liver diseases can now be treated, such as hepatitis B, hepatitis C, Wilson disease, etc. Control of the primary liver disease can lead to fibrosis regression4 and improvement in liver function to the point where long-term survival without liver transplantation is possible.5
Pathogenesis of Ascites in Cirrhosis
The development of cirrhotic ascites is the end result of physiologic compensatory steps that eventually lead to failure of other body systems.6 The increased resistance to blood flow through the cirrhotic liver leads to a progressive, increase in production of vasodilatory substances, such as nitric oxide, in an attempt to minimize resistance (Fig. 6.1). This leads to progressive splanchnic arterial dilation (arterioles arising from the celiac, superior mesenteric, and inferior mesenteric arteries). The combination of increased resistance and increased flow results in increased pressure in the portal venous system, that is portal hypertension. Increased hydrostatic pressure from portal hypertension, in combination with decreased oncotic pressure from reduced liver synthesis of albumin, results in increased Starling forces. This allows increased transudation of albumin-poor plasma from the liver and mesentery into the peritoneal cavity. Ascites accumulates when the rate of transudation into the peritoneal cavity exceeds the rate of lymphatic drainage from the peritoneal cavity.
Fig. 6.1 Pathophysiology of portal hypertension: peripheral arteriolar vasodilatation hypothesis. Gradual increase in portal hypertension over years leads to ascites that becomes more difficult to control. ADH, antidiuretic hormone; GFR, glomerular filtration rate.
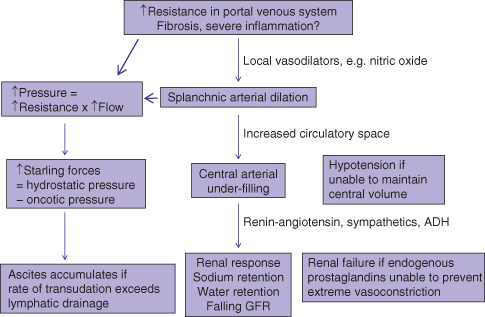
In addition, simultaneous splanchnic arterial dilation leads to an overall increased vascular space and under-filling of the central arterial compartment. To compensate, the kidneys retain sodium and water, resulting in a larger total plasma volume. As both the cirrhotic process and increase in liver resistance progress with time, there is progressive splanchnic arterial dilation leading to greater stimulation of the renin–angiotensin system, sympathetic nervous system, and release of antidiuretic hormone, causing vasoconstriction in the kidneys and even more sodium retention, further worsening ascites control (Table 6.1). These compensatory mechanisms eventually fail, resulting in renal failure from renal ischemia and circulatory failure from under-filling of the central arterial space.
Table 6.1 Progression of cirrhosis leads to ascites that becomes more refractory to treatment
| Phase | Ascites control | Description |
| 1 | Subclinical | Sodium retention is mild and most of dietary sodium intake can be cleared by the kidneys. The mildly increased fluid transudate is cleared by the lymphatics and so clinical ascites does not develop. Severe sodium loading, for example after intravenous fluid resuscitation for a variceal bleed, can lead to transient ascites. |
| 2 | Diet control | Sodium retention is moderate but high sodium intake can lead to ascites accumulation whereas a dietary sodium restriction can still lead to resolution of ascites. |
| 3 | Diuretics | Renal sodium restriction is much lower (<10 mEq/day) due to increased stimulation of the renin–angiotensin system, the sympathetic nervous system, and production of ADH. Dietary sodium restriction alone will not prevent ascites accumulation. Despite increasing renal vasoconstriction, GFR is maintained due to local production of prostaglandins. Blockage of prostaglandins through NSAIDs can lead to renal failure. |
| 4 | HRS-2 | Serum creatinine > 1.5 mg/dL or GFR has fallen to <40 mL/min due to renal vasoconstriction and all other causes of renal dysfunction have been excluded, no improvement after at least 2 days of diuretic withdrawal and plasma volume expansion with albumin. Ascites control becomes more difficult despite diuretics. Prognosis for survival is poor: 50% at 5 months and 20% at 1 year. |
| 5 | HRS-1 | Rapidly progressive renal failure to >2.5 mg/dL (221 µmol/L) within 2 weeks, usually in the setting of pre-existing HRS-2. Frequently precipitated by physiologic stress: major surgery, gastrointestinal bleeding, sepsis such as spontaneous bacterial peritonitis. Prognosis for survival is very poor: 20% at 2 weeks. |
Management of ascites: dietary sodium restriction to <2 g/day is effective for up to phase 2. Diuretics are required from phase 3 onwards, with higher doses of diuretics required to phase 4. By phase 4, diuretics may not work adequately and therapeutic paracentesis may be needed. However, survival is not improved by paracentesis—a transplant is needed to improve prospects for long-term survival.
ADH, antidiuretic hormone; GFR, glomerular filtration rate; HRS, hepatorenal syndrome; NSAID, non-steroidal anti-inflammatory drug.
Ascites is best detected by ultrasound during routine ultrasound surveillance for liver cancer in the patient with known cirrhosis. In those with unsuspected cirrhosis, ascites volume must exceed 1.5 L before bulging flanks are evident. Shifting dullness is reported to have 83% sensitivity and 56% specificity for the detection of ascites, but the performance characteristics depend on the volume of ascites, with massive ascites being obvious on clinical exam and small ascites essentially undetectable without ultrasound.7
Paracentesis to remove ascites is safe (see below for discussion of what tests should be done of ascites fluid). The complication rate of the procedure is low—approximately 1% for abdominal wall hematomas. Serious complications, such as bowel perforation, are rare, and the rate is estimated at less than 1/1000 procedures. Clotting factors such as plasma are not required prior to paracentesis, even if the INR is elevated to 9. The sites used for paracentesis are the left or right lower quadrant, approximately 3 cm cephalad and 3 cm medial to the anterior superior iliac spine. The right side is less optimal if the cecum is dilated as may occur with chronic lactulose use. Bedside ultrasound guidance is useful, especially if the clinician is not confident of the site for paracentesis.
Diagnostic paracentesis should be done for all patients with ascites. A serum to ascites albumin gradient (SAAG) more than 11 g/L is supportive of the diagnosis of cirrhotic ascites (Tables 6.2 and 6.3). The white blood cell (WBC) count and differential must always be evaluated on ascites fluid, where a finding of more than 250 neutrophils/µL (0.25 × 109 neutrophils/L) is supportive of infection. If infection is suspected because of low-grade fever, abdominal pain, or elevated leukocyte count from baseline, ascites fluid should be cultured in blood culture bottles as this increases the yield of a positive culture. Antibiotics (third-generation cephalosporin or amoxicillin–clavulanic acid i.v.) should be started if the fluid neutrophil count is elevated—do not wait for results of the culture as they may not be available for 24 hours. Infection is usually caused by Gram-negative bacteria from normal intestinal flora. Although routine culture of ascites fluid is not necessary, it should be done if the ascites WBC is more than 250 neutrophils/µL. Diagnostic paracentesis should be repeated if there is no clinical improvement after 48 hours of antibiotics. If the ascites WBC and neutrophils remain high (<25% fall from baseline), a change in antibiotics, taking into account local antibiotic resistant strains, should be considered. After recovery from an episode of spontaneous bacterial peritonitis, secondary prophylactic antibiotics (norfloxacin 400 mg/day or ofloxacin 400 mg/day, or Septra 750 mg once weekly indefinitely) should be prescribed.
Table 6.2 Routine tests of ascites fluid for uncomplicated ascites
| Tests | Interpretation |
| Albumin | SAAG = serum albumin – ascites albumin SAAG > 11 g/L is consistent with cirrhotic ascites and portal hypertension plus second cause of ascites formation |
| Cell count and differential | PMN ≥ 250 cells/µL (0.25 × 109/L) supports diagnosis of spontaneous bacterial peritonitis Empiric therapy with a third-generation cephalosporin × 5 days should be started, but can be modified based on results of bacterial culture/sensitivity testing |
PMN, polymorphonuclear cells; SAAG, serum ascites albumin gradient.
Table 6.3 Special tests of ascites fluid
| Indication | Tests | Interpretation |
| Suspect infection | Bacterial culture in blood culture bottle | Positive cultures can guide antibiotic therapy; positive in 50% |
| Intestinal perforation | PMN extremely high Multiple organisms Protein > 10 g/L Glucose < 3.0 mmol/L ALP > 240 IU/L CEA > 5 ng/mL | Search for site of perforation |
| Peritoneal carcinomatosis, ovarian carcinoma, gastric carcinoma | Cytology | Rarely positive in other cancers such as primary liver cancer |
| High risk for tuberculous peritonitis: endemic area, HIV | Mycobacterial culture | Sensitivity only ∼50% |
| Chylous (milky) fluid | Triglycerides > 100 mg/dL | Although possible with cirrhotic ascites, rule out lymphatic obstruction or lymphatic disruption |
ALP, alkaline phosphatase; CEA, carcinoembryonic antigen; PMN, polymorphonuclear cells.
Management of Cirrhotic Ascites
Given the pathogenesis of cirrhotic ascites, therapy is directed at minimizing sodium intake and diuretics to enhance sodium excretion if necessary (Table 6.1). This strategy will not work for those with SAAG less than 11 (ascites not associated with cirrhosis) and is likely to result in renal insufficiency. Once the diagnosis of cirrhotic ascites is established, the most important long-term management strategy is to treat all treatable underlying liver diseases. Alcohol consumption should be minimized, hepatitis B should be controlled, and so forth. In cases where the underlying cause of liver disease is not yet effectively treatable (e.g. fatty liver), ascites control will become increasingly more difficult and will eventually require liver transplantation.
Sodium Restriction
Dietary sodium intake should be restricted to 2000 mg/day (88 mmol/day). Maintenance intravenous fluids should be given with caution as they can be significant sources of sodium intake. For example, 1 liter of normal saline contains 154 mmol sodium, almost 2 days worth of dietary sodium intake. More stringent restriction is not recommended because food becomes less palatable and more difficult to find, making malnutrition a significant risk. As long as the daily urinary excretion of sodium exceeds 88 mmol/day, ascites should decrease and resolve (phase 2). If ascites seemingly cannot be controlled by sodium restriction, urine biochemistry should be performed. If a random urinary sample yields a sodium level greater than potassium or a 24-h urine collection shows very high levels of sodium excretion (>100 mmol/day), then high dietary sodium intake is the likely cause of ascites accumulation. Fluid restriction is usually not necessary as water loss passively follows sodium loss. Chronic hyponatremia correlates with the severity of cirrhosis but on its own is seldom morbid unless the hyponatremia is corrected too rapidly.
Diuretics
Once the daily urinary excretion of sodium falls below 78 mmol/day, ascites will accumulate despite sodium restriction (phase 3). Diuretics can increase urinary sodium excretion. The usual approach is to introduce a combination of oral diuretics, typically furosemide 40 mg and spironolactone 100 mg daily. Diuretics are advised to be taken in the morning—night time dosing will lead to nocturia and disturbed sleep. Weight loss should be limited to no more than 0.5 kg/day if there is no underlying edema. When diuretics are first required, the response is usually dramatic and ascites quickly resolves. Even if ascites resolves, the Model for End-stage Liver Disease (MELD) should be calculated and a referral for liver transplantation assessment be made if MELD is elevated to the criteria of your local transplant center (e.g. MELD ≥15 for Toronto in 2011). If the underlying liver disease is untreated and cirrhosis progresses, urinary sodium excretion continues to fall and higher doses of diuretics are required. Before raising the doses of diuretics, it is worthwhile reviewing dietary sodium restriction. Adequate naturesis can be checked by testing the urine; a random urine sodium/potassium ratio more than 1 or a 24-h urine sodium more than 78 mmol/day suggests adequate diuresis with current doses of diuretics but inadequate dietary sodium restriction. The doses of diuretics can be increased, trying to maintain the ratio of furosemide 40 mg to spironolactone 100 mg, in steps of 40 mg and 100 mg, respectively, to a maximum dose of furosemide 160 mg and spironolactone 400 mg once daily in the morning (i.e. not divided doses). As the doses of diuretics required increase, the serum creatinine also increases, and is suggestive of type 2 hepatorenal syndrome when serum creatinine is more than 133 µmol/L (Box 6.1).8 Spironolactone can cause tender gynecomastia, which can be significant. Amiloride (10–40 mg) can be used to as a substitute for spironolactone but, in general, it is less effective than spironolactone. Careful monitoring of electrolytes and creatinine should be done (at least monthly) after starting diuretics and after each change in dose of diuretics. Diuretics should be held and reassessed if there are unexpected increases in fluid loss (vomiting, diarrhea) or if complications of uncontrolled/recurrent encephalopathy, significant hyponatremia (Na <120 mmol/L) despite fluid restriction, or renal insufficiency (creatinine >133 µmol/L) develops (Box 6.1).








