- Country of birth is the most important risk factor for HBV infection.
- Age at acquisition is strongly associated with the risk of chronic infection (95% of infants develop chronic infection, 5% of immunocompetent adults develop chronic infection).
- Patients progress through the clinical stages of chronic hepatitis B (CHB) over their lifetime (immunotolerant, immune clearance, immune control, HBeAg-negative chronic hepatitis B).
- Treatment is indicated in the immune clearance phase, chronic HBeAg-negative hepatitis B with active disease, prior to receiving immunosuppressive therapy, and possibly in other select circumstances.
- Treatment options include peginterferon or nucleoside/nucleotide analogues.
- Hepatocellular carcinoma may develop in the setting of CHB infection even in the absence of cirrhosis. Screening for hepatocellular carcinoma in patients with CHB is based on age, sex, ethnicity, family history, and the presence of cirrhosis.
- HDV may complicate HBV with coinfection or superinfection. HDV worsens the course of both acute and chronic HBV infection and there are limited treatment options.
Background
Hepatitis B virus (HBV) has a unique genomic structure and lifecycle. Despite being a DNA virus, HBV has a very compact genome of just 3.2 kb, much smaller than either HIV or hepatitis C virus (HCV) (both ∼ 9.6 kb). The genomic DNA of HBV is partially double stranded, meaning that the inner (positive) strand of the DNA is shorter than the outer (negative) strand. HBV infects hepatocytes and upon entering the nucleus of the cell, the inner strand is completed by cellular polymerases to form so-called covalently closed circular or cccDNA. cccDNA serves as the template for HBV replication but also remains in infected cells for the life of the cell, possibly even surviving cell replication. The fact that small amounts of cccDNA remain in hepatocytes indefinitely has important implications for the possibility of reactivation of HBV even after long-term quiescence.
Epidemiology
Approximately 2 billion people have been exposed to HBV infection (i.e. test anti-HBc positive) worldwide and nearly 400 million remain chronically infected (HBsAg positive). The prevalence varies widely with the highest rates occurring in east (China, Taiwan) and southeast Asia (Vietnam, Cambodia, Laos, Korea) as well as sub-Saharan Africa. In highly endemic countries, prevalence of HBsAg ranges from at least 8% to as high 20% of the adult population. However, exposure to HBV is much more common with up to 80% of adults testing positive for the presence of anti-HBc in endemic regions.
Intermediate endemic countries (prevalence 2–8%) include South Asia (India, Pakistan), eastern and southern Europe (Italy, Turkey, Greece), the Middle East and most of South America. Low endemic countries (prevalence <2%) include North America (excluding the Canadian North), western and northern Europe, Australia, and New Zealand. However, large urban centers in low endemic countries may have intermediate prevalence of HBV due to large foreign-born populations (e.g. New York, London, Toronto).
Risk Factors
HBV is spread parenterally via blood and body fluids and is the most infectious of the blood-borne chronic viral infections. HBV is not spread by saliva, insect bites, or by casual contact. The age at acquisition largely determines the outcome of HBV infection. Approximately 95% of neonates infected with HBV will develop chronic infection. In contrast, 95% of immunocompetent adults will spontaneously clear infection (with or without symptomatic acute infection). Rates of chronicity are higher in people with HIV infection and other causes of immunosuppression (Table 13.1). Vertical and very early horizontal transmission account for the vast majority of chronic HBV infections globally and hence country of birth is by far the most important risk factor for chronic HBV infection.
Table 13.1 Risk factors for HBV
| Vertical transmission | Most common route of transmission worldwide |
| Early horizontal transmission | Precise mechanisms unclear; important that infants are vaccinated within 12 hours of birth if mother or father/siblings HBsAg positive |
| Sexual | 95% spontaneous clearance |
| Injection drug use | High rate of spontaneous clearance |
| Blood transfusion | 1 in 60 000 in USA |
| Hemodialysis | Immunosuppressed patients are more likely to become chronically infected |
Vertical transmission generally occurs at delivery, with very low rates of intrauterine infection. Cesarean section has not been shown to reduce the rates of transmission. The risk of vertical transmission depends on the viral load and HBeAg status of the mother. Transmission to infants occurs in up to 90% of cases in which the mother is HBeAg positive because of the associated high viral loads (generally >10E7 IU/mL). In contrast, chronic infection occurs in less than 30% of infants born to HBeAg-negative mothers. In addition to the viral titer, the presence of HBeAg itself increases the risk of chronicity.
Early horizontal transmission occurs in children under the age of 5, particularly in sub-Saharan Africa. The direct modes of transmission are unknown. To prevent early horizontal transmission, it is important that children with known HBV-positive close contacts (siblings, father, other relatives) should also be vaccinated at birth. Other sources of transmission include: sexual (hetero- and homosexual), injection drug use, blood transfusion (1 in 60 000 in USA), hemodialysis, and other parenteral exposures.
Postexposure Prophylaxis
Infants of known HBsAg-positive mothers should receive HBV vaccine (10 µg) and hepatitis B immune globulin (HBIG) (0.06 mL/kg) at birth (within 12 hours) to reduce rates of transmission. These infants should then complete a full three or four-course vaccine schedule (1 and 6 months) or (1, 2, and 12 months). Even with appropriate vaccination and HBIG administration, transmission may occur in 5 to 10% of cases, particularly if mothers are HBeAg-positive and have viral loads above 10E7 IU/mL. It is important to explain to HBsAg-positive mothers that vaccination and HBIG are not 100% effective. For women with very high viral loads (>10E7 IU/mL), antiviral therapy with lamividuine, telbivudine or tenofovir in the final trimester of pregnancy may reduce the risk of transmission.
All infants must be tested at 9–12 months of age. Testing should not be done prior to 9 months as antibodies may still come from the mother prior this age.
Infants of mothers of unknown HBsAg status should receive HBV vaccine, but not HBIG, within 12 hours of birth, and complete a full HBV vaccination schedule. Infants of HBsAg-positive fathers (or other household members) should be vaccinated at birth (no HBIG) to prevent early horizontal transmission.
Unvaccinated individuals exposed to HBV should receive HBV vaccine and HBIG within 48 hours of exposure. Vaccinated individuals exposed to HBV should have HBsAb titers assessed. Those with anti-HBs titers less than 10 IU/mL should be treated as unvaccinated individuals. If protective titers (>10 IU/mL) are present, no action is required.
Serology
A number of serological markers have been identified that indicate different stages of HBV infection. The definition of each marker is described below and is shown in Table 13.2.
- HBsAg indicates ongoing infection. Still present even with complete viral suppression with therapy (the HBsAg protein is produced before DNA replication).
- Anti-HBs indicates immunity to HBV unless HBsAg also present (up to 10% of cases). May be acquired by vaccination or immune control of natural infection (if accompanied by anti-HBc).
- Anti-HBc (IgG) indicates exposure to HBV. All chronically infected (HBsAg-positive) will also be anti-HBc positive. Lone anti-HBc positive status may be a false positive if there are no risk factors for exposure. Anti-HBc generally remains positive life-long.
- Anti-HBc (IgM) indicates either acute infection or flare of chronic HBV infection.
- HBeAg is a soluble protein produced by the virus that is not necessary for viral replication. Its presence is almost always associated with active viral replication and high viral load; however, the corollary is not true, i.e. absence of HBeAg does not imply low-level replication. HBeAg is also thought to act as a toleragen, helping HBV evade the host immune response. HBeAg crosses the placenta and likely tolerizes the fetus to HBV prior to birth, increasing the risk of chronic infection.
- Anti-HBe indicates clearance of HBeAg (although both HBeAg and anti-HBe may be present simultaneously). The presence of anti-HBe is generally associated with lower viral loads than the HBeAg-positive state; however, active liver disease may be present.
- HBV DNA: direct measurement of viral particles in the blood. The titer of HBV DNA ranges vary widely, from less than 1 to more than 10 log IU/mL. The degree of liver disease associated with a given viral titer depends on the stage of disease (see below).
Table 13.2 Interpretation of viral markers in hepatitis B
| Test | Full name | Significance |
| HBsAg | Hepatitis B surface antigen | Ongoing HBV infection |
| Anti-HBs | Anti-hepatitis B surface antibody | Immunity (natural or vaccine induced) |
| Anti-HBc (IgG) | Anti-hepatitis B core antibody (IgG) | Indicates current or resolved HBV infection |
| Anti-HBc (IgM) | Anti-hepatitis B core antibody (IgG) | Acute HBV infection or flare of chronic HBV |
| HBeAg | Hepatitis B e antigen | Active viral replication |
| Anti-HBe | Anti-hepatitis B e antibody (may persist after clearing HBsAg) | May indicate immune control but active viral replication may still occur |
| HBV DNA | Hepatitis B DNA | Direct measurement of viral particles: range 1–10 log IU/mL |
Stages of Infection
Most chronically infected individuals are infected at birth or in early childhood and progress through a series of stages of infection over their lifetime. Although the stages are shown in the order in which they typically occur, patients may flip-flop between stages. The serological markers help determine the stage of infection; however, for a given patient, at a given moment, it may be difficult to determine the current stage of infection. As a result, longitudinal follow-up is necessary to clarify the stage of disease and pattern of progression for individual patients.
Adult-acquired HBV that becomes chronic may have a different natural history. The immunotolerant phase of infection is typically shorter and often does not occur at all, with patients moving directly into the immune clearance phase with active HBeAg-positive hepatitis. A similar scenario may occur in babies or young children infected from an HBeAg-negative mother or any other HBeAg-negative contact. Because of the absence of the tolerizing effects of the HBeAg protein, the risk of severe acute or even fulminant HBV infection is greater in infections occurring from an HBeAg-negative mother/contact.
Immunotolerant Phase
Profile.
HBeAg positive, normal alanine aminotransferase (ALT), very high viral load (>8 log IU/mL), and normal liver biopsy (if performed).
The immunotolerant phase is the initial stage of infection, typically lasting from birth into the third or fourth decade of life. However, in certain regions of the world, particularly sub-Saharan Africa, the immunotolerant phase may be much shorter, lasting only into the early teenage years. Differences in the duration of this phase of infection relate to the HBV genotype and possibly to host factors as well.
The immunotolerant phase is characterized by very high levels of viremia with no associated liver damage. HBV is not a cytopathic virus, with liver damage occurring due to the immune response targeting the virus. During this stage of infection, the virus is not recognized by the immune system and hence no liver damage ensues.
Management
During the immunotolerant phase of infection, therapy of all types is ineffective and also is unnecessary as there is no progressive liver disease. Therapy of immunotolerant patients is not currently advised and carries a high risk of leading to antiviral resistance. Patients who remain in the immunotolerant phase of infection until above age 40 may have an increased risk of hepatocellular carcinoma. Whether therapy is warranted for such patients is unknown.
Immune-Active/Immune-Clearance Phase
Profile.
HBeAg positive, anti-HBe negative, elevated ALT (range from mild elevation to into the 1000s), and active inflammation with or without fibrosis on liver biopsy.
All patients eventually transition from the immunotolerant phase of infection to the immune-active/clearance phase. The transition typically occurs in the third or fourth decade of life, but may occur in childhood or early adolescence. The precipitant(s) for entering this active phase of HBV infection is unknown. Some patients remain in the immunotolerant phase until later in life and this is likely associated with an increased risk of development of hepatocellular carcinoma (HCC).
With immune recognition, flares of hepatitis occur as the immune system attempts to clear infected hepatocytes. The immune-active phase is characterized by recurrent flares of disease, with rises in ALT, accompanied by falls in viral load. Ultimately, patients eventually clear HBeAg and develop anti-HBe antibodies; however, the process of HBeAg seroconversion may take months to years to develop. The repeated flares of hepatitis lead to progressive liver disease with associated fibrosis and may ultimately progress to cirrhosis. Rarely, flares may be very severe, leading to jaundice and occasionally to liver failure. However, most flares are entirely asymptomatic, even with marked ALT elevation, and thus all patients require regular follow-up for their recognition, particularly those with ongoing viral replication. Symptomatic flares are more likely to lead to spontaneous HBeAg seroconversion. Patients may intermittently be positive or negative for both HBeAg and anti-HBe, particularly as they are transitioning from the immune-active to immune-control phase of infection.
Management
Before instituting therapy, patients should be followed for at least 3 to 6 months to allow for spontaneous HBeAg seroconversion. If the ALT remains elevated with elevated HBV DNA for longer than 6 months, therapy should be considered. Prior to starting therapy, a liver biopsy or non-invasive assessment of fibrosis is generally advised to clarify the degree of liver damage. Exclusion of other causes of liver disease is also important in the appropriate clinical scenario (alcohol, fatty liver, HCV coinfection, HDV etc.).
Immune Control
Profile.
HBeAg negative, anti-HBe positive, HBV DNA less than 2000 IU/mL, ALT normal, and liver biopsy (if performed) shows minimal activity with variable degrees of fibrosis depending on previous course of infection.
The phase of immune control is sometimes referred to as the “inactive carrier state”; however, this term is not preferred because patients may or may not remain inactive and all require long-term follow-up. Notably, some patients may even have developed cirrhosis during the previous HBeAg-positive stage of infection. Although many patients truly remain inactive lifelong, 40% of patients will go on to develop HBeAg-negative chronic hepatitis B.
The level of HBV DNA is useful for predicting subsequent short-term course:
- HBV DNA less than 2000 IU/mL, risk of flare in 12 months less than 10%—follow annually;
- HBV DNA 2000–20 000 IU/mL, risk of flare in 12 months 30–40%—follow every 6 months;
- HBV DNA more than 20 000 IU/mL, risk of flare in 12 months 70%—follow closely and consider antiviral therapy.
For patients with low or undetectable HBV DNA levels, HBsAg should be checked annually, as 0.5–1% of patients will clear HBsAg per year. HBsAg clearance prior to age 50 is associated with an excellent prognosis and, in the absence of cirrhosis, follow-up is no longer required. HBsAg clearance after age 50 is still associated with a risk of HCC and therefore ongoing ultrasound surveillance is recommended. Disease activity may flare significantly with immunosuppression (see below) but can be largely prevented with the use of pre-emptive antiviral therapy.
Management
In the immune-control phase, the immune system is adequately controlling viral replication with no ongoing liver damage. Provided the liver disease remains quiescent, no treatment is indicated; however, age and ethnicity-appropriate screening for HCC is indicated. Treatment should be considered prior to significant immunosuppression (e.g. cancer chemotherapy, organ transplantation, therapy with biologics) to avoid reactivation (see below).
HBeAg-Negative Chronic Hepatitis B
Profile.
HBeAg negative, anti-HBe positive or negative, HBV DNA more than 2000 IU/mL, elevated ALT, and liver biopsy with activity and variable fibrosis.
Because HBeAg is not necessary for viral replication, even after HBeAg clearance, viral replication may occur at high levels and this can lead to an associated hepatitis and progressive liver disease. HBeAg-negative chronic HBV is sometimes referred to as “pre-core mutant HBV” because many patients harbor virus with a mutation leading to a stop codon in the pre-core gene that is responsible for production of HBeAg. “HBeAg-negative CHB” is a preferable term because mutations other than the pre-core mutation may occur, most commonly in the promoter for the HBeAg gene (basic core promoter). In either scenario, viral replication occurs despite the absence of HBeAg. Mutations leading to the loss of HBeAg (pre-core or basic core promoter) do not impair viral replication.
Importantly, HBV-related liver disease may occur at much lower thresholds of viral replication, that is lower HBV DNA titers, because unlike during the immunotolerant phase, the immune system recognizes and responds to HBV and tolerizing effects of HBeAg are absent. Therefore, even small rises in HBV DNA (between 2000 and 20 00 IU/mL) can lead to significant ALT flares and disease progression.
The course of HBeAg-negative CHB is highly variable. Some patients have persistently elevated ALT with detectable viral titers; however, others may have only intermittent rises in ALT. Similar to the immune-active phase, almost all flares of ALT are entirely asymptomatic. If increases in ALT are observed but HBV DNA remains below 2000 IU/mL, other causes of liver disease should be investigated (e.g. fatty liver disease, alcohol, HDV). Unlike during the immunotolerant phase, with HBeAg-negative CHB, higher titers of HBV DNA are associated with an increased risk of progression of liver disease and development of HCC. Antiviral therapy reduces the risk of disease progression and likely lowers the risk of HCC in patients.
Management
If there are intermittent or persistent increases in ALT associated with HBV DNA levels above 2000 IU/mL, antiviral therapy should be considered. Antiviral resistance is generally less of a problem than in patients with HBeAg-positive disease because of the lower viral loads at the start of therapy. Prior to starting therapy, a liver biopsy or non-invasive assessment of fibrosis is generally advised. Exclusion of other causes of liver disease is important in the appropriate clinical scenario (alcohol, fatty liver, HCV coinfection, HDV, etc.).
Assessment of Liver Fibrosis
HBV is a very dynamic disease and, although treatment is very effective, in many patients it is difficult to stop therapy once it has been instituted. A high percentage of patients will also control their infection/disease with no therapy. The presence of fibrosis is a risk factor for further disease progression and, as a result, most guidelines and experts recommend assessing the degree of fibrosis, either with liver biopsy or non-invasive techniques, prior to starting therapy. In patients with no evidence of fibrosis, therapy can usually be delayed but regular (at least annual) follow-up is required.
Liver biopsy remains the gold standard for assessing the degree of liver fibrosis; however, it is an invasive procedure with a small risk of complications and patients do not like having multiple biopsies, as is often required to manage this very dynamic disease. Fortunately, in recent years, non-invasive tests of liver fibrosis—both serum and elastography tests—have been developed. Non-invasive assessments of fibrosis correlate fairly well with liver biopsy but sensitivity and specificity vary by method (see Chapter 2).
Options for non-invasive assessment of liver fibrosis include: serum markers, transient elastography, and radiographic imaging.
Serum Markers
Numerous panels of serum markers have been developed to predict liver fibrosis, which correlate relatively well with liver biopsy findings. Most panels were developed for HCV infection and are generally less predictive in HBV, because of the more dynamic nature of the disease. Serum panels are shown in Chapter 2, with performance characteristics in HCV. In addition to formal serological panels, some evidence of advanced fibrosis can often be gleaned from routine laboratory tests.
- Thrombocytopenia (<150 000/µl) often indicates cirrhosis with portal hypertension.
- AST/ALT ratio: ALT is typically higher than aspartate aminotransferase (AST) in chronic HBV infection. With advanced fibrosis, the AST is often higher than ALT.
- Hypergammaglobulinemia: polyclonal increases in IgG are suggestive of advanced liver fibrosis caused by gut bacteria bypassing the liver.
Transient Elastography
Transient elastography (TE) uses ultrasound or magnetic resonance to estimate the liver stiffness. Increased liver stiffness is associated with advanced fibrosis. Like other non-invasive tools, TE is very good at extremes of fibrosis, thus it is very accurate at identifying no fibrosis or cirrhosis. However, TE is less precise with intermediate levels of fibrosis. TE may be significantly influenced by the presence of hepatic inflammation, making it relatively unreliable during flares of hepatitis, with a tendency to overestimate the degree of liver fibrosis. Similarly, TE may be very high during acute HBV infection due to hepatic inflammation rather than fibrosis.
- Advantages of TE: non-invasive, rapid, performed in office setting, correlates well with liver biopsy, may predict clinical outcome (variceal bleeding, ascites, etc.).
- Disadvantages of TE: poor accuracy during acute flares of hepatitis and in obese patients or those with ascites, no histological information regarding etiology.
Radiographic Imaging
Although in certain instances, ultrasound or other imaging of the liver may be useful diagnostically, imaging of all types is neither sensitive nor specific for the presence of progressive liver fibrosis. Many patients with bridging fibrosis or even early, well-compensated cirrhosis will have a normal-appearing liver on imaging tests. The presence of signs of portal hypertension (splenomegaly, varices, ascites) in the setting of HBV infection are indicators of advanced liver disease, but unfortunately the absence of such features is not adequate to exclude significant hepatic fibrosis.
HBV Therapy
Therapy for HBV has improved dramatically in the past decade with the development of potent, well-tolerated, oral antivirals. Therapy for HBV may be divided into two overall approaches: direct-acting antivirals (nucleoside/nucleotide analogues) and immune stimulation (interferon).
Nucleoside/Nucleotide Analogues
Although HBV is a DNA virus, it has an unusual replication cycle involving an RNA intermediate. From the DNA template, viral RNA is transcribed. The viral RNA serves as the template for reverse transcription to make new HBV DNA. Because of the presence of “reverse transcriptase” activity of the HBV polymerase, many of the reverse transcriptase inhibitors developed for HIV have proven effective at inhibiting HBV replication. HBV encodes only one enzymatically active protein (the polymerase) and as a result all HBV inhibitors target the same stage in replication, namely reverse transcription. To date, all HBV antivirals are nucleoside or nucleotide analogues (NAs). NAs mimic the natural nucleoside/nucleotide and compete for incorporation into nascent virions. Incorporation of the NAs leads to chain termination with production of non-functional, incomplete viral DNA.
Although NAs have much greater affinity for the viral polymerase, they can theoretically inhibit host DNA polymerases as well. Inhibition of the γ DNA polymerase of mitochondrial DNA can lead to mitochondrial toxicity with lactic acidosis. Fortunately, mitochondrial toxicity is very rare with all NAs used for HBV monoinfection. The lactic acidosis syndrome has been reported to occur in patients with advanced liver disease with use of entecavir. Fortunately, side effects of any kind are uncommon with the NAs. Patients tolerate all the oral therapies well. Adefovir and tenofovir may cause dose-related renal toxicity and therefore careful follow-up of renal function is important with these agents.
There are three classes of HBV NAs based on differing structures, resistance patterns, and side-effect profiles.
- L-nucleosides: lamivudine, telbivudine, emtricitabine
- variable potency but identical resistance profile;
- resistance to one confers resistance to all in this class;
- notably, resistance occurs more frequently with less-potent agents; lamivudine resistance occurs at a rate of approximately 15% per year with 70% resistance by 5 years, while resistance to telbivudine, a more potent agent, occurs at a rate of 7% per year;
- minimal toxicity.
- variable potency but identical resistance profile;
- Acyclic phosphonates: adefovir, tenofovir
- potency of adefovir limited by dose (10 mg daily) but good resistance profile (29% at 5 years);
- resistance to adefovir greater if pre-existing lamivudine resistance (22% at 2 years);
- tenofovir is very potent and no resistance reported to date;
- adefovir-resistant mutants are sensitive to tenofovir;
- renal toxicity reported for both agents; ranges from asymptomatic rise in creatinine with or without drop in phosphate to Fanconi-like syndrome with phosphaturia, amino aciduria, and rarely to renal failure;
- metabolic bone disease (osteomalacia and osteoporosis) has also been reported with prolonged use, presumably due to phosphate wasting.
- potency of adefovir limited by dose (10 mg daily) but good resistance profile (29% at 5 years);
- Cyclopentane: entecavir
- very potent;
- very good resistance profile in NA-naïve patients (1.4% at 4 years);
- resistance rate much higher if pre-existing lamivudine resistance (15% at 3 years);
- double dose (1 mg daily) recommended if pre-existing lamivudine resistance;
- minimal toxicity; rare reports of lactic acidosis syndrome in patients with decompensated cirrhosis.
- very potent;
A comparison of efficacy for HBeAg-positive patients with 1 year of therapy is as follows:
- HBsAg loss: 3% tenofovir, 2% entecavir, less than 1% other NAs;
- HBeAg seroconversion: approximately 15–20% for all NAs;
- HBV DNA suppression: 70–80% for potent agents (tenofovir/entecavir), 42% for lamivudine, 20% for adefovir;
- ALT normalization: 70% for potent agents (tenofovir/entecavir), 60% for lamivudine, 48% for adefovir;
A comparison of efficacy for HBeAg-negative patients with 1 year of therapy is as follows:
- HBsAg loss: rare with all agents;
- HBV DNA suppression: 80–90% for potent agents (tenofovir/entecavir/telbivudine), 60–70% for lamivudine, 50–60% for adefovir;
- ALT normalization: 80–90% for potent agents (tenofovir/entecavir), 60–70% for lamivudine, 60% for adefovir.
Duration of Therapy with Nucleoside/Nucleotide Analogues
For patients with HBeAg-positive disease, treatment should be continued until HBeAg seroconversion (loss of HBeAg and development of anti-HBe) occurs or resistance develops. Even with prolonged HBV DNA suppression to undetectable levels, if HBeAg remains positive patients will universally relapse upon stopping therapy. Once HBeAg seroconversion occurs, treatment should be continued for at least 6 months of consolidation. Recent evidence suggests that even with consolidation therapy, rates of HBeAg seroreversion and HBeAg-negative CHB following cessation of NA therapy are high (up to 70%) and therefore some experts suggest continuing therapy until loss of HBsAg is achieved. Unfortunately, because HBsAg loss is an uncommon event, particularly with NA therapy (and almost only seen in those infected with genotypes A and D), such an approach may lead to very prolonged therapy with associated increased costs and risks of long-term toxicity.
For patients with HBeAg-negative CHB, duration of NA therapy is less clear. Most data suggest that even after prolonged suppression of HBV DNA to undetectable levels, relapse is very common upon stopping therapy. Continuing therapy until HBsAg clearance is reasonable; however, HBsAg loss is very uncommon in patients who start NA for HBeAg-negative CHB, meaning this approach may lead to exceedingly prolonged treatment.
Because of the need for prolonged therapy once therapy is initiated, it is critical to institute therapy only in those who truly need it.
Interferon/Peginterferon
As an alternative to the NAs, which are direct HBV antivirals, interferon and peginterferon may also be used to treat HBV. Interferon is an important component of the innate antiviral immune response. Interferon acts by activating a signaling cascade leading to the activation of numerous antiviral genes within infected cells. Interferon also activates the adaptive immune response.
Although standard interferon is effective, pegylated interferon (peginterferon) is more commonly used because of its more convenient, once-weekly dosing schedule. There have been no trials comparing standard to pegylated interferon for the treatment of HBV infection and as a result either option may be used. Although some patients do not respond to interferon, no true interferon-resistant HBV has been identified.
The major issue with interferon is tolerability. Many side effects are reported with the most common being flu-like symptoms (myalgias, arthralgias, fatigue, low-grade fever), mild bone marrow suppression, and depression. Stimulation of the immune system may worsen or bring out latent autoimmune conditions. For unclear reasons, patients with HBV tend to tolerate interferon better than those with HCV infection with lower rates of depression and other side effects reported.
Interferon or peginterferon are given for 6 to 12 months. Patients with high ALT and low HBV DNA at baseline are more likely to respond to interferon-based therapy. Viral genotype may also be important with patients with genotypes A and B infection responding much better than those with either genotype D or C. HBsAg titer, if available, may be useful for predicting response to peginterferon. Patients without a decline in HBsAg titer by 12 weeks are very unlikely to respond and therapy can likely be discontinued.
HBeAg seroconversion occurs in 25–30% of patients after 1 year of therapy and appears to be very durable (>70%) over long-term follow-up. Because interferon is immune stimulatory, ALT flares are common during therapy. ALT flares often occur immediately prior to HBeAg seroconversion. Treatment should be continued with ALT flares unless there is evidence of hepatic synthetic dysfunction (jaundice, coagulopathy, etc.). Although synthetic dysfunction with peginterferon is rare, it is reported and thus peginterferon should be avoided in patients with cirrhosis with any evidence of even mild synthetic dysfunction. Importantly, interferon leads to HBsAg clearance in 7–10% of patients, even up to 3–4 years after cessation of therapy, higher than reported for any of the NAs.
Combination Therapy
Although combination therapy sounds attractive based on data from other diseases (e.g. HIV), there is minimal evidence to support a benefit to combination therapy in HBV among treatment-naïve patients. All NAs have the same viral target (HBV DNA polymerase) and therefore they compete with each other at the active site of the enzyme. There is no additive or synergistic antiviral effect of combination NA therapy. There may be a benefit in terms of delaying the emergence of resistance; however, this has not been clearly demonstrated. Combination NA therapy is preferable to sequential monotherapy for documented HBV resistance (i.e. add a second agent rather than switch to another agent, particularly for lamivudine resistance treated with adefovir). A “switch” approach may be reasonable with the newer, more potent agents. Combination therapy with lamivudine and peginterferon is not superior to either therapy alone. Whether combination with the more potent NAs and peginterferon would be beneficial is unknown.
Management of NA Resistance
Viral breakthrough is defined as an increase in HBV DNA titer by 1 log above the nadir achieved on therapy. Prior to modifying therapy or diagnosing resistance, it is critical to check with the patient about compliance. Up to 30% of viral breakthrough is related to non-compliance rather than resistance.
If the patient reports compliance, recheck the HBV DNA in 1 month. If the titer is still rising, resistance is likely present. Ideally, confirmation with resistance testing is helpful, however it is not widely available. Once resistance is suspected/confirmed, therapy should be modified, even if ALT remains normal:
- For L-nucleoside resistance (lamivudine/telbivudine/emtricitabine)
- add adefovir
- switch to tenofovir or tenofovir plus emtricitabine (Truvada)
- switch to entecavir (use 1 mg daily, i.e. double dose and cost).
- add adefovir
- For adefovir resistance
- add L-nucleoside (lamivudine/telbivudine)
- switch to entecavir
- switch to tenofovir or tenofovir plus emtricitabine (Truvada).
- add L-nucleoside (lamivudine/telbivudine)
- For entecavir resistance
- switch to tenofovir or tenofovir plus emtricitabine (Truvada).
- For tenofovir resistance
- switch to entecavir.
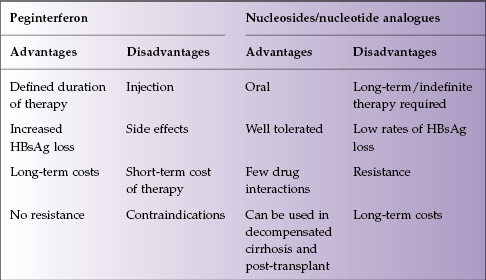
The advantages and disadvantages of peginterferon therapy and NA therapy are presented in Table 13.3.
Table 13.3 Advantages and disadvantages of interferon versus nucleosides/nucleotide analogues
Management in Resource-Limited Settings
Specialized testing, including HBV DNA levels, may not be available in resource-limited areas of the world, many of which have a high prevalence of HBV infection. Lack of access to testing complicates therapy somewhat but, in most instances, appropriate decisions can be made with relatively simple tests. In patients with HBeAg-positive disease, ALT and complete blood count (CBC) can be followed every 6 months with institution of therapy if the ALT increases and does not return to normal (i.e. spontaneous HBeAg seroconversion) within 6 months. In those with active, HBeAg-negative disease, recognition that HBV is the cause of elevated liver tests is more difficult. If liver biopsy is available, it can be quite useful, particularly if there are other possible etiologies for liver inflammation such as fatty liver disease. If liver biopsy is not available, but ALT is persistently elevated in a HBsAg-positive, HBeAg-negative patient, institution of therapy would be appropriate, using improvement of ALT as an imperfect assessment of efficacy. In HBsAg-positive, HBeAg-negative patients with clinical or biochemical evidence of advanced disease (impaired liver synthetic function, low platelets, etc.), the threshold for institution of therapy should be very low, even in the absence of availability of HBV DNA testing.
Once on therapy, if HBV DNA testing is unavailable, ALT, CBC, and creatinine should be followed at 3-monthly intervals. Any ALT elevation should be investigated appropriately with a detailed history and physical examination (medication history including herbal remedies, compliance with HBV therapy, exposures to coinfection, etc). If the ALT elevation persists in a compliant patient, drug resistance should be considered and managed as described above. Notably, ALT flares may be associated with HBeAg seroconversion with both interferon and NA therapy. Therefore in HBeAg-positive patients, before resistance is diagnosed, HBeAg serology should be re-evaluated. Creatinine testing is necessary both to diagnose NA toxicity (with adefovir or tenofovir) and to make dosing adjustments in patients with renal impairment.
In patients on NA therapy with normal ALT, HBeAg and HBsAg should be checked annually. If HBsAg clearance occurs, therapy can be discontinued, but patients must be followed as reactivation with re-emergence of HBsAg has been described.
For all patients, HIV testing is mandatory prior to starting any therapy (see below).
HBV Reactivation
Liver disease related to HBV infection is immune mediated and thus the immune status of the host has a major impact on the course of disease. Patients with inactive disease may have severe or even fatal reactivation of disease in the setting of immunosuppression. Reactivation is most commonly reported in patients receiving cytotoxic cancer chemotherapy but has also been reported with prolonged courses of corticosteroids and more recently with biologics used for immune-mediated diseases (rheumatoid arthritis, inflammatory bowel disease). Up to 50% of patients with inactive disease (HBeAg negative, normal ALT, low or undetectable HBV DNA, but still HBsAg positive) will have a flare of HBV-related hepatitis with standard chemotherapy. Typically, HBV DNA titers rise during therapy and the flare of hepatitis occurs following immune reconstitution. A high index of suspicion is necessary to identify HBV reactivation because in patients receiving chemotherapy, many other potential causes for ALT elevation exist. If HBV reactivation occurs, antiviral therapy is indicated; however, in severe cases hepatitis may progress despite therapy. Fortunately this scenario can largely be prevented with the use of pre-emptive antiviral therapy with lamivudine (or likely any NA). Small, randomized controlled trials have shown that using pre-emptive antiviral therapy is more effective than starting therapy only if reactivation occurs. Pre-emptive therapy prevents severe reactivations, which may lead to liver failure and will likely interrupt and thus compromise chemotherapy. If treatment is delayed until the ALT begins to rise, therapy may not be effective. Treatment should be continued for 6 months after the end of all immunosuppression but patients should be followed to ensure that a treatment withdrawal flare does not occur.
Prevention of reactivation CHB (induced by immune suppression).
Because of the potential serious consequences of HBV reactivation and the presence of very effective therapy, the CDC recently recommended that all patients scheduled to receive cytotoxic chemotherapy be screened for HBV (at least HBsAg).
Anti-HBc Alone
The term “resolved” infection for individuals who are anti-HBc positive but HBsAg-negative is somewhat of a misnomer because of the presence of the low levels of HBV (in the form of cccDNA) remaining in the liver. With severe immune suppression, even patients who are HBsAg-negative can experience HBV reactivation. Reappearance of HBsAg is usually referred to as “reverse seroconversion”. The greatest risk for reverse seroconversion is with allogeneic bone-marrow or stem-cell transplant (up to 50% incidence) but it may also occur with advanced HIV disease and it has been reported to occur in up to 25% of patients receiving rituximab-based chemotherapy. Pre-emptive antiviral therapy is indicated for anti-HBc-positive patients undergoing bone marrow or stem-cell transplant. Whether antiviral therapy would be helpful in the other scenarios, particularly with rituximab therapy, is currently unknown. If therapy is not initiated, HBsAg should be followed regularly with institution of therapy if it becomes positive. The risk of reactivation with standard solid tumor chemotherapy is very low and prophylaxis is not indicated.
Indications for Therapy in Chronic Hepatitis B
Even with ongoing viral replication, therapy for CHB is not always indicated. The indications for which treatment is recommended are discussed below.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







