Minimally Invasive Nonhysteroscopic Methods for Endometrial Ablation
Michael S. Baggish
During the 1990s and continuing to the present time, several techniques have been described using various devices to perform blind ablation of the endometrium. The basis for these methods revolved around the idea of (a) removing the skill factor as a variable for endometrial ablation; (b) eliminating the requirement for a distending medium and hence any risks attributed to the distending medium; (c) simplifying the ablative operation to its lowest common denominator and permitting the procedure to be performed in an office setting; (d) shortening the time to perform the ablation relative to the standards set by the most expert hysteroscopic surgeons performing the procedure via laser or electrosurgery hysteroscopically (i.e., under direct vision); and (e) obtaining equivalent or near equivalent results to those obtained hysteroscopically.
Interestingly, many of the pioneers of these techniques were the same individuals who had previously pioneered hysteroscopic ablation procedures. As with all new techniques, long-term follow-up of sufficient numbers of cases is required to determine the true worth of the operation.
For convenience, these techniques may be divided into the following categories:
Balloon techniques
Hot water with in situ heating
Monopolar electric current via multiple electrodes mounted on the balloon
Nonballoon hot saline
Computerized, continuously circulating in situ heated saline
Externally heated saline delivered by hysteroscope
Nonballoon electrosurgical
Microwave
Bipolar
Cryosurgical
Chemical and photochemical
A thorough preoperative workup prior to ablation by any of these techniques is absolutely necessary. This should include a preoperative diagnostic hysteroscopy to visualize the endometrium followed by directed sampling of the endometrium. For patients with abnormal uterine bleeding, a basic hematology workup should include lab tests for bleeding and clotting factors.
Many of the techniques that are discussed below share a similar early history with hysteroscopic endometrial ablation: a lack of published data about animal and in vitro studies, as well as little or no data on objective histologic efficacy studies based on in vivo research of women who subsequently underwent hysterectomy after the experimental ablation. Additionally, there is scant literature detailing safety studies. Few of the techniques listed above have provided peer review published data about in vitro, objective histologic efficacy, and safety studies. In contrast, a number of clinical studies now appear in the literature describing favorable results reminiscent of the early published data about the efficacy of endometrial ablation. Unfortunately, this splendid efficacy historically tends to be pared down as time and usage march on. As with most procedures, early caution usually pays off. The rush to the marketplace frequently results in reverse progress over the long-term test of time. Perino et al. (2004) recently compared endometrial laser intrauterine thermal therapy (ELITT) with endometrial resection in a randomized trial with 58 patients in each cohort. At 36 months, the amenorrhea rate was 61% for ELITT and 24% for resection. No significant complications were recorded in either group.
The first technique investigated was described in 1990 by Phipps et al. A probe was placed in the uterus and attached to a radio frequency generator emitting electrothermal energy in the megahertz range (Menostat) (Fig. 27.1). The treatment averaged approximately 15 minutes with temperatures of 60°C to 65°C. Sharp et al. (1995) reported data on 23 women who were treated by this technique. All patients were pretreated with gonadotropin-releasing hormone (GnRH) agonist, and endometrial height was measured by vaginal ultrasound. Patients were followed for 6 months postoperatively.
Amenorrhea was reported in 57% of the cases, light menstruation in 26%, and failure in 12%. No complications were reported. In contrast, several complications occurred during the original investigation, some very serious in the form of thermal burns secondary to high-frequency leaks. Indeed the complex maneuvers required to protect the patient from these types of injuries (e.g., keeping the patient free of any conductive contact [metal, operating room table, intravenous pole, speculum, electrocardiogram electrodes, pulse oximeter]) are major drawbacks of this technique (Fig. 27.2). Tulandi and Felemban (2001) described the appearance of the uterine cavity before and after microwave ablation in 62 women. Seven women had a small island of intact endometrium, and the cavity was distorted in six secondary to a submucous myoma.
Amenorrhea was reported in 57% of the cases, light menstruation in 26%, and failure in 12%. No complications were reported. In contrast, several complications occurred during the original investigation, some very serious in the form of thermal burns secondary to high-frequency leaks. Indeed the complex maneuvers required to protect the patient from these types of injuries (e.g., keeping the patient free of any conductive contact [metal, operating room table, intravenous pole, speculum, electrocardiogram electrodes, pulse oximeter]) are major drawbacks of this technique (Fig. 27.2). Tulandi and Felemban (2001) described the appearance of the uterine cavity before and after microwave ablation in 62 women. Seven women had a small island of intact endometrium, and the cavity was distorted in six secondary to a submucous myoma.
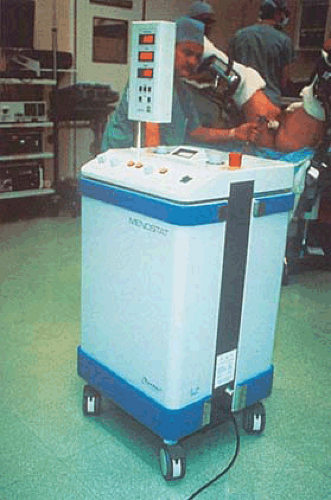 FIGURE 27.1 Microwave control unit operating in the megahertz range (foreground), and the microwave probe is inserted into the patient’s uterus (background). |
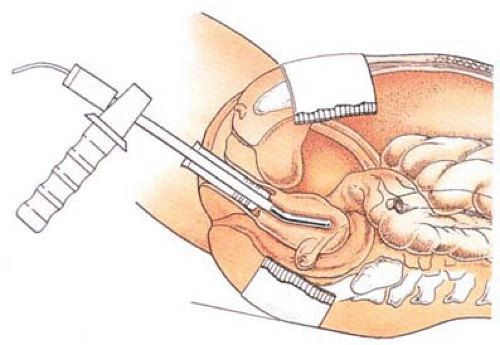 FIGURE 27.2 The microwave probe heavily insulated from the surrounding vagina is placed in the uterus for endometrial ablation. |
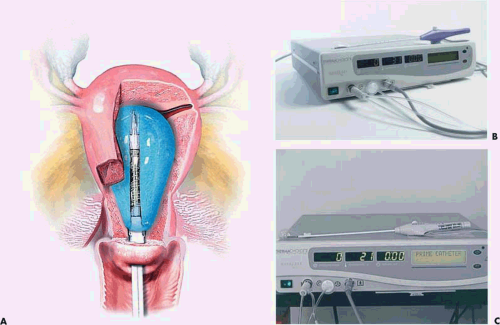
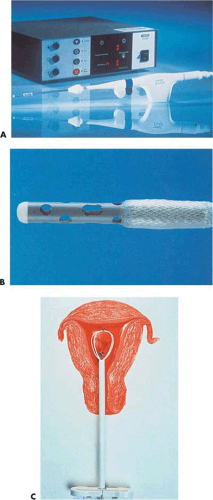
Neuwirth et al. (1994) reported an endometrial ablator using a balloon into which sterile water was placed (Fig. 27.3A–C). A heating element within the distended balloon raised the water temperature to approximately 90°C. The balloon also contained thermistors, which recorded temperature within the balloon (Fig. 27.4A, B). Six patients were studied to determine efficacy and safety of the technique by subjecting the uterus to thermal ablation followed by hysterectomy. During the procedure, endometrial and serosal temperatures were recorded. Gross examination and histology were performed. No pathologic alterations were observed on the hematoxylin and eosin sections. Four additional patients were studied at another center. In these cases, NADH diaphorase staining was performed and showed destruction ranging in depth from 3.3 to 5 mm.
A subsequent paper by Singer et al. reported a clinical trial on 18 women with abnormal bleeding, of whom 7 reported subsequent light bleeding and eight reported spotting or amenorrhea. Three patients subsequently underwent hysterectomy or hysteroscopic resection. Loffer and Grainger (2002) reported a 5-year follow-up comparing uterine balloon with ball electrode ablation. One hundred and forty-seven of the original 255 women were evaluated. Twenty-five patients underwent hysterectomy or other procedures. Ninety-five percent of the balloon and 97% of the ball ablation patients reported normal or less bleeding. Watermeyer and Amso evaluated the reasons for the low amenorrhea rate following balloon ablation via intraoperative and postoperative ultrasound monitoring. They determined a thin endometrium predicts successful ablation and the varying position of the balloon may affect outcome. Leung et al. (2003) hysteroscopically evaluated the endometrial cavity following balloon ablation finding adhesions, fibrosis, and obliteration. A few women had “normal appearing” cavities. Unfortunately, this study was deficient because no sampling and histologic studies were performed.
Baggish et al. (1995) reported data on five in vivo sheep and 32 freshly excised human uteri using a computer-controlled, continuously circulating, continuously heated small volume of saline (10 to 15 mL) within the uterine cavity (Fig. 27.5). As with the preceding technique, the thermal cannula within the uterine cavity continuously monitored intrauterine pressure and, by means of thermistors, measured intrauterine temperature. Similarly, infrared techniques and thermocouples measured uterine serosal, tubal, and cervical temperatures (Fig. 27.6). The temperatures of the saline ranged from 65°C to 90°C with 80% of the specimens treated at 80°C to 85°C for 15 minutes. All specimens were evaluated grossly and then stained with hematoxylin and eosin, as well as NADH diaphorase (Fig. 27.7). All sheep specimens treated at 70°C showed endometrial and superficial myometrial destruction. All human uteri including those with cavity deformity caused by submucous myomas, which were treated with 80°C for 15 minutes, showed destruction of the endometrium and destruction below the endometrium into the myometrium extending 1 to 3 mm (Fig. 27.8). The peak serosal temperatures ranged from 35°C to 49°C. The mean intrauterine pressure was 26 mm Hg. Both the Neuwirth and Baggish articles showed pictures of the histologic effects of the hot liquid on the endometrium and myometrium.
Bustos-Lopez et al. (1998) reported an experience using the same technique of hot saline ablation in 11 women undergoing hysterectomy (Fig. 27.9). These in vivo safety studies monitored intrauterine pressure and serosal, multiple tubal, cervical, and peritoneal temperatures. No tubal spill of saline was observed. Thermal damage to a depth of 2 to 4 mm (mean 3 mm) was observed by NADH diaphorase
staining in every case (Fig. 27.10). Serosal and tubal temperatures during treatment ranged from 29°C to 40°C. The 40°C temperatures were recorded at the right and left cornu.
staining in every case (Fig. 27.10). Serosal and tubal temperatures during treatment ranged from 29°C to 40°C. The 40°C temperatures were recorded at the right and left cornu.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree