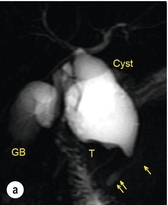
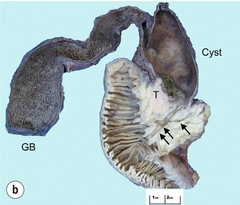
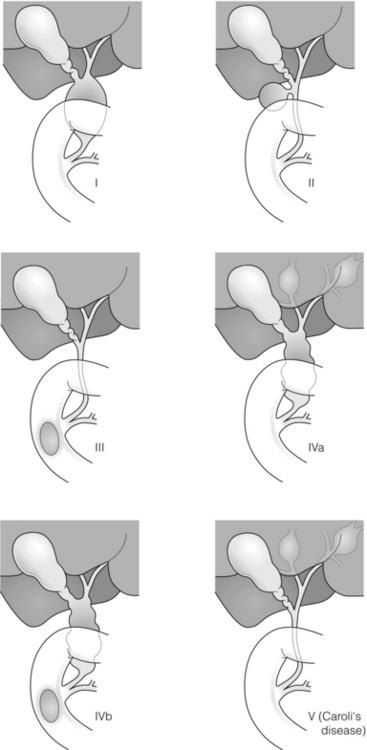
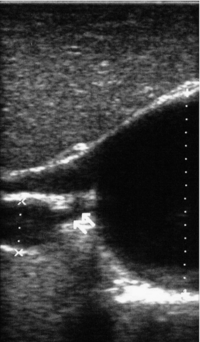
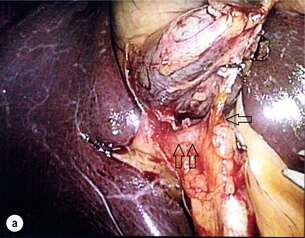
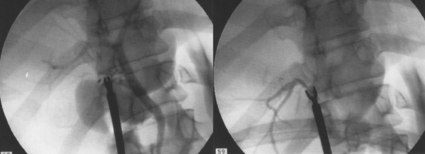
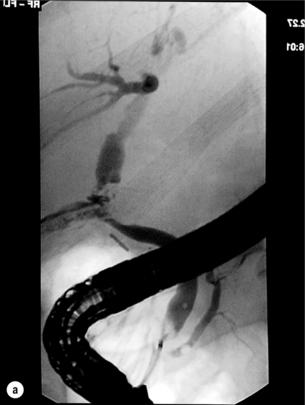
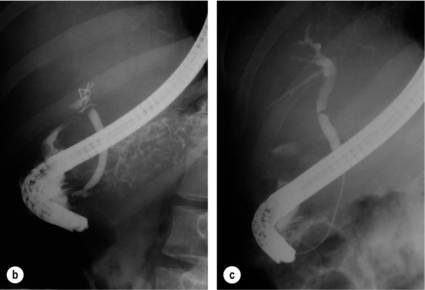
11 Apart from those disorders related to choledocholithiasis, benign diseases of the biliary tree are relatively uncommon (Box 11.1). The most challenging issues are in patients who present with symptoms associated with biliary strictures, which arise more commonly following iatrogenic injury during cholecystectomy. Congenital abnormalities such as choledochal cysts and biliary atresia are usually in the domain of the paediatric surgeon, although later presentation of cysts may occur after missed diagnosis or when revisional surgery is required. Most of the published literature regarding benign non-gallstone biliary disease is retrospective or at best prospectively gathered, non-randomised data, but clear guidelines can be followed based upon observation. Presentation is usually in the early neonatal period with prolongation of neonatal jaundice. Most patients are treated in specialist neonatal surgical units; however, occasionally patients may be referred to adult units for assessment for liver transplantation following previous unsuccessful treatment. Management in the neonate is by porto-enterostomy (Kasai’s operation), which involves anastomosis of a Roux limb of jejunum to the tissue of the hilum. Restoration of bile flow has been reported in 86% of infants treated before 8 weeks of age, but only 36% in older children.1 Four-year survival is dependent on the timing of surgery. Of 349 North American children with biliary atresia, 210 (60%) required later liver transplantation, with a 4-year transplantation survival of 82%.2 Recent evidence has suggested better outcomes following maternal liver-related liver transplantation, potentially due to tolerance to non-inherited maternal antigens.3 The earliest description of a choledochal cyst was by Douglas in 1952,4 who described a 17-year-old girl with jaundice, fever and a painful mass in the right hypochondrium. However, presentation is usually in childhood and around 25% are diagnosed in the first year, although prenatal diagnosis is now possible with improvements in antenatal ultrasonography. Adult centres treat a small proportion of those presenting with delayed diagnosis as well as those with complications from previous cyst surgery. The incidence of choledochal cysts in Western countries is around 1 in 200 000 live births but it is much higher in Asia. There is frequent association with other hepatobiliary disease such as hepatic fibrosis, as well as an aberrant pancreatico-biliary duct junction.5 Magnetic resonance cholangiopancreatography (MRCP) now allows images that are superior to traditional cholangiography (Fig. 11.1), and it should be recommended due to its non-invasive nature.6 Figure 11.1 MRCP (a) and macroscopic photograph (b) demonstrating a type I choledochal cyst with a distal cholangiocarcinoma in a 42-year-old Caucasian woman requiring a pancreaticoduodenectomy. Gallbladder (GB), tumour (T), pancreatic duct (single arrow) and aberrant common channel (double arrow) are shown. Courtesy of Professor Prithi S. Bhathal, Pathology Department, University of Melbourne, Australia. The modified Todani classification is employed to describe the various forms of choledochal cyst7 (Fig. 11.2). Type I, the most common, represents a solitary cyst characterised by fusiform dilatation of the common bile duct. Type II comprises a diverticulum of the common bile duct, whilst type III cysts are choledochocoeles. Type IV is the second most common, with extension of cysts into the intrahepatic ducts. Lastly, type V involves intrahepatic cystic disease with no choledochal cyst, which merges into the syndrome of Caroli’s disease. Figure 11.2 Modified Todani classification for choledochal cysts.7 Reproduced from Todani T, Watanabe Y, Narusue M et al. Congenital bile duct cysts: classification, operative procedures, and review of thirty-seven cases including cancer arising from choledochal cyst. Am J Surg 1977; 134:263–9. With permission from Elsevier. In the Western literature, the incidence of cholangiocarcinoma is reported to be approximately 12% (Fig. 11.1),8 compared to Todani et al.’s Japanese experience of 16% in 1353 patients.9 The incidence of malignancy is reported to be 2% at 20 years, increasing to 43% for those in their sixties.10 Cyst drainage without cyst excision does not prevent later malignant change, and there is continuing debate regarding the precise ongoing risk following cyst resection. Takeshita et al. reported 180 patients who underwent primary surgery for a choledochal cyst. Synchronous malignancy was found in 36 patients (20%), with only one of the remaining 144 patients developing malignancy during follow-up.11 During operative exposure, intraoperative ultrasound is very useful to identify the biliary confluence, the intrahepatic extension of the cyst, and the relationship to the right hepatic artery above and to the pancreatic duct below (Fig. 11.3). Small aberrant hepatic ducts may enter the cyst below the biliary confluence and these are missed frequently on preoperative imaging.12 Such aberrant ducts are usually identified once the cyst has been opened. The cyst is normally best excised in its entirety and this is facilitated by opening it along its anterior length. This aids identification of the vessels from which the cyst is freed. Early identification of the biliary confluence aids the surgeon in planning the incorporation of any segmental duct into the eventual hepatico-jejunal Roux-en-Y anastomosis. Dissection into the head of the pancreas is made easier by use of bipolar scissors and the CUSA™ (ultrasonic surgical aspiration system, ValleyLab, Boulder, CO) if the plane of dissection is obscured by fibrosis or inflammation. It may be necessary to leave a small oversewn lower common bile duct stump to avoid compromise to the pancreatic duct lumen; however, recurrent pancreatitis and possible malignant transformation remain possible complications. Figure 11.3 Operative ultrasound scan of a type I choledochal cyst. The junction of the undilated proximal biliary tree with the cyst (long dotted line) is demonstrated. The right hepatic artery is posterior (two arrows), as is the right branch of the portal vein (short dotted line). The true incidence of biliary injury following laparoscopic cholecystectomy remains obscure. It has been suggested that there was a slight increase in the incidence of injuries following initial introduction of the laparoscopic technique,13 with a reported incidence of 0.3–0.7%.14–16 Open cholecystectomy is said to have a lower incidence of biliary injury, with a rate of 0.13%.17 Recent variations in technique such as single-incision laparoscopic surgery (SILS) cholecystectomy are not immune to biliary injury. Han et al. recently reported two (1.5%) bile duct injuries in 150 patients having single-port laparoscopic surgery.18 Previous reports of injury during laparoscopic cholecystectomy suggested that injury was more likely to occur when performed for pancreatitis, cholangitis or acute cholecystitis.19 However, in a prospective analysis of patients referred following biliary injury, 71% occurred in patients in whom the indication for cholecystectomy was biliary colic alone,20 and thus surgeons should always be vigilant regardless of the indication. Many techniques have been described to decrease the risk of injury to the common bile duct during cholecystectomy. The main risk factors are thought to be inexperience, aberrant anatomy and inflammation.19,21 However, in an analysis of 252 laparoscopic bile duct injuries, the authors suggested that the primary cause of error was a visual perceptual illusion in 97% of cases, whilst faults in technical skill were thought to have been present in only 3% of injuries.22 Correct identification of the biliary anatomy is essential in avoiding injury to the extrahepatic bile duct. Dissection of Hartmann’s pouch should start at the junction of the gallbladder and cystic duct and continue lateral to the cystic lymph node, thus staying as close as possible to the gallbladder. The biliary tree and hepatic arterial anatomy is highly variable and therefore great care must be taken in identifying all structures within Calot’s triangle before ligation. In Couinaud’s published study of biliary anatomy, 25% had drainage of a right sectoral duct directly into the common hepatic duct.23 Sometimes this structure may follow a prolonged extrahepatic course, where it can be at greater risk from cholecystectomy. The right hepatic artery may also course through this area. All structures should be traced into the gallbladder to minimise the risk of injury (Fig. 11.4 and 11.5). Figure 11.4 Aberrant biliary anatomy. The normal biliary anatomy is a trifurcation of the right sectoral and left hepatic ducts forming the common hepatic duct which receives the cystic duct after a variable distance. Operative photograph (a) and a cholangiogram (b) of a short cystic duct (single arrow) draining into the right posterior sectoral duct (double arrow), which has a long extrahepatic course. Figure 11.5 Operative cholangiography of an aberrant right sectoral duct. The injury was recognised after division of the duct following cholangiography. The cholangiogram catheter was used to obtain a cholangiogram of the aberrant duct. The surgeon obtained advice by telephone and a decision was made to ligate the duct. The patient remains asymptomatic. Calot’s original description of gallbladder anatomy described a triangle formed by the cystic duct, common hepatic duct and superior border of the cystic artery.24 For satisfactory visualisation of the structures, dissection should also extend above the cystic artery to the liver. Extensive dissection should be avoided in Calot’s triangle as diathermy injury may occur to the lateral wall of the common hepatic duct. Furthermore, arterial bleeding in this area should not be cauterised or clipped blindly. Most bleeding can be controlled with several minutes of direct pressure with a laparoscopic forceps compressing Hartmann’s pouch on to the bleed point. During the era of open cholecystectomy many advocated complete excision of the cystic duct to its insertion into the common bile duct to avoid a cystic duct stump syndrome. However, extensive dissection around the common bile duct with or without diathermy may cause an ischaemic stricture due to damage to the intricate blood supply of the common hepatic duct. Many authors argue that operative cholangiography is essential to avoid biliary injury.16,19 Fletcher et al. reported an overall twofold reduction in biliary injuries with the use of operative cholangiography, with an eightfold decrease in complex cases.19 Flum and colleagues analysed retrospectively the Medicare database in the USA and identified 7911 common bile duct injuries following cholecystectomy. After adjusting for patient-level factors and surgeon-level factors the relative risk was 1.49 when intraoperative cholangiography was not used.16 When the use of intraoperative cholangiography has undergone cost analysis, routine cholangiography has been found to be the most cost-effective during high-risk operations when employed by less experienced surgeons.25 Unfortunately, many operative cholangiograms are interpreted incorrectly and injuries are missed. Although this event should be less frequent with the use of modern C-arm imaging, in reported series of biliary injuries only 6–33% of operative cholangiograms are interpreted correctly.20,26 For correct anatomical interpretation of the proximal biliary tree, both right sectoral/sectional ducts and the left hepatic duct should be visualised. In the presence of an endoscopic sphincterotomy, contrast will preferentially flow into the duodenum and the patient may need to be placed in a head-down position to fill the intrahepatic ducts. If the anatomy is unclear no proximal clip should be placed on what is presumed to be the cystic duct, to avoid a crush injury to what may be the common hepatic duct. Retrograde cholecystectomy has been described previously as a safe technique when inflammation around Calot’s triangle makes identification of the anatomy difficult. Nonetheless, care still needs to be exercised during dissection to avoid injury to the right hepatic artery and common hepatic duct, which may be adherent to an inflamed gallbladder. Eight such patients have recently been described by Strasberg and Gouma.27 If identification remains impossible then the gallbladder can be opened to facilitate identification of the cystic duct. A subtotal cholecystectomy should be considered if a safe plane of dissection cannot be established, thus avoiding injury to the common hepatic or left hepatic ducts. Originally described for open cholecystectomy, these techniques have now also been performed laparoscopically.28 Injury to the distal biliary tree is less technically demanding to repair than involvement of the biliary confluence. The success of reconstruction depends on the type of injury and the anatomical location.29 Bismuth first described a classification system for biliary strictures reflecting the relationship of the injury to the biliary confluence (Table 11.1).30 Strasberg et al. further proposed a broader classification to include a number of biliary complications, including cystic stump leaks, biliary leaks and partial injuries to the biliary tree (Fig. 11.6).17 Table 11.1 Bismuth classification of biliary strictures Figure 11.6 Strasberg classification. Type A injuries include leakage from the cystic duct or subvesical ducts. Type B involves occlusion of part of the biliary tree, most usually an aberrant right hepatic duct. If the former injury involves transection without ligation this is termed a type C injury. A lateral injury to the biliary tree is a type D injury. Type E injuries are those described by Bismuth and subdivided into his classification (Table 11.1). Adapted from Strasberg SM, Hertl M, Soper NJ et al. An analysis of the problem of biliary injury during laparoscopic chole cystectomy. J Am Coll Surg 1995; 180:102–25. With permission from the American College of Surgeons. Ligation of sectoral ducts may cause subsequent or late atrophy of the drained liver segments, which may become infected secondarily. Occasionally liver resection or transplantation may be required for fulminant hepatic failure secondary to combined biliary and vascular injuries.31,32 More commonly, injuries may present late with secondary biliary cirrhosis, which may require liver transplantation when liver failure results.31,32 In many patients there is a delay until referral, despite evidence of a biliary injury. In a report by Mirza et al., the median interval until referral was 26 days.33 This delay is not inconsequential as the opportunity for an early repair is lost and results in the liver sustaining further damage. In a review by Carroll et al., only 27% of patients underwent a successful repair by the primary surgeon responsible for the injury, whilst 79% of repairs performed following referral had a successful outcome.34 If experienced help is not at hand, no attempt should be made to remedy the situation since this may compromise subsequent successful management. A T-tube or similar drain should be placed to the biliary injury and drains left in the subhepatic space, followed by referral to a specialist centre. No attempt should be made to repair a transection or excision of the bile duct. A partial injury to the bile duct may sometimes be managed by direct closure with placement of a T-tube through a separate choledochotomy. Primary repair with or without a T-tube for complete transection of the common bile duct is nearly always unsuccessful. This may result from unappreciated loss of common duct or an associated arterial injury, or result from local diathermy injury or devascularisation of the duct from overzealous dissection of the common bile duct35 (Fig. 11.7a,b). Succesful endoscopic treatment is possible for failed primary repair; however, as many as 32% will require subsequent hepatico-jejunostomy.36 Figure 11.7 (a) Failure of primary repair with T-tube. Primary repair was performed for an injury to the common bile duct presenting with biliary peritonitis. A T-tube was inserted through the anastomosis and this was removed at 4 weeks. An anastomotic stricture developed and the patient required a hepatico-jejunostomy 2 months later. (b) Failure of primary repair for ligation of the common bile duct. A complete transection of the common bile duct identified at postoperative endoscopic retrograde cholangiopancreatography (ERCP). Immediate repair was performed with a direct duct-to-duct repair. (c) A tight anastomotic stricture is demonstrated at a later ERCP. Further assessment depends on the clinical situation. The majority of biliary fistulas are due to leaks from the cystic duct stump or subvesicle ducts, and endoscopic retrograde cholangiopancreatography (ERCP) allows anatomical definition, endoscopic sphincterotomy or stent placement. As complete transection of the bile duct precludes ERCP, computed tomography intravenous cholangiography (CT-IVC) or MRCP can determine continuity of the biliary tree prior to endoscopy. Occasionally, persistent bile drainage is associated with choledocholithiasis requiring sphincterotomy and stone extraction. Most simple cystic duct stump leaks can be resolved by endoscopic stenting if cannulation is possible at ERCP37 and occasionally side injury to the biliary tree can be controlled with endoscopic stent placement.38 ERCP will identify a stricture or complete transection of the bile duct; however, identification of complete transection with MRCP will avoid the risks of an unnecessary ERCP. Overzealous instillation of contrast should be avoided, and placement of an endoscopic stent should only be considered after consultation with a specialist unit since this may introduce sepsis into the biliary tree and compromise further management. Furthermore, an undrained biliary tree may allow proximal biliary dilatation, thereby facilitating later reconstruction. Although some have reported satisfactory resolution of biliary strictures with endoscopic stenting alone, the follow-up has usually been short and almost all patients require later surgery in our experience. Partial occlusion of the duct by a clip may be remedied by balloon dilatation with or without placement of a stent; however, delay in diagnosis may result in subsequent recurrent stricture formation. Nonetheless, de Reuver et al. reported 110 patients with bile duct strictures following cholecystectomy that were treated with endoscopic stenting, 48 (44%) of which had already undergone attempted surgical repair. At a mean follow-up of 7.6 years, 74% of patients had a successful outcome.37 The development of removable endoscopic expandable metal stents has recently been described, although long-term results and large series are not yet available. Furthermore stent migration can complicate treatment.
Benign biliary tract diseases
Introduction
Congenital anomalies
Choledochal cysts
Classification
Risk of malignancy
Special operative techniques
Iatrogenic biliary injury
Aetiology
Techniques to avoid injury

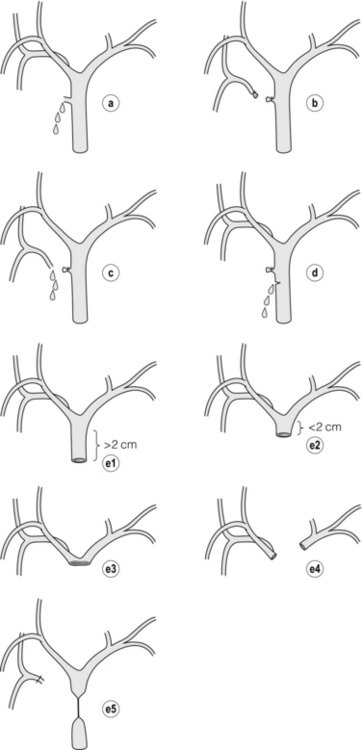
Classification
Bismuth classification
Definition
Bismuth 1
Low common hepatic duct stricture – hepatic duct stump > 2 cm
Bismuth 2
Proximal common hepatic duct stricture – hepatic duct stump < 2 cm
Bismuth 3
Hilar stricture with no residual common hepatic duct – hepatic duct confluence intact
Bismuth 4
Destruction of hepatic duct confluence – right and left hepatic ducts separated
Bismuth 5
Involvement of aberrant right sectoral hepatic duct alone or with concomitant stricture of the common hepatic duct
Presentation
Management
Postoperative recognition: biliary fistula
Postoperative recognition: biliary obstruction






