Chapter 101 Whole-organ pancreas and pancreatic islet transplantation
Overview
Type 1 diabetes mellitus, formerly known as juvenile diabetes, is characterized by hyperglycemia as a result of the nearly complete destruction of the insulin-producing β-cells of the pancreatic islets of Langerhans. The loss of β-cells is the result of a T-cell–mediated autoimmune attack that typically occurs during childhood or early adolescence. Insulin replacement can lead to acceptable control of blood glucose levels; however, affected individuals are subject to the development of various secondary complications that include cardiac disease, stroke, retinopathy and blindness, nephropathy and renal failure, peripheral and autonomic neuropathy, and amputation (Atkinson & Eisenroth, 2001). Although tight glycemic control has been shown to decrease the number of diabetes-related secondary complications, it also has been shown to lead to an increased number of dangerous hypoglycemic episodes (Diabetes Control and Complications Trial [DCCT] Research Group, 1993).
Whole-Organ Pancreas Transplantation
History and Early Results
On December 20, 1893, P. Watson Williams grafted three pieces of a sheep pancreas into the subcutaneous tissues of a diabetic child, who died 3 days later of unrelenting diabetic ketoacidosis (Williams, 1894). This first attempt to treat diabetes with transplantation, although unsuccessful, preceded decades of animal experimentation, in which investigators developed the methods necessary to perform a vascularized pancreas transplant; pancreas transplantation was subsequently used as a model to study diabetes and glucose homeostasis.
The first clinical vascularized pancreas transplantation was performed on December 17, 1966, by William Kelly and Richard Lillehei at the University of Minnesota. The patient had temporary insulin independence but eventually required graft removal and ultimately died of postoperative complications (Kelly et al, 1967). The subsequent early experience with pancreas transplantation at Minnesota and at a few other centers was characterized by some technical success, but no graft functioned beyond 1 year, and the enthusiasm for this procedure dwindled.
Recipient Operation
In two areas of pancreas transplantation, current-day techniques differ: in the drainage of exocrine secretions and the venous drainage of the graft. Exocrine drainage is performed either via the intestinal tract or via the urinary tract. Throughout most of the 1980s and 1990s, drainage of the pancreatic secretions into the recipient bladder was the most common form of exocrine drainage. This technique is convenient for monitoring organ function by measurement of amylase levels in the urine; however, problems with hematuria, cystitis, bicarbonate loss, and dehydration are all associated with bladder drainage. These complications necessitate surgical revision to enteric drainage in up to 20% of bladder-drained pancreas recipients (Stratta, 2005). Based on these issues, and on the lower rejection rates observed with newer immunosuppressive medications, the majority of transplant centers now perform enteric drainage of the exocrine secretions. This enteric drainage is either directly into a loop of jejunum in a side-to-side fashion or into a Roux-en-Y limb of jejunum.
The venous drainage of the graft is either to the systemic circulation, via an iliac vein or the inferior vena cava, or to the portal circulation. Portal venous drainage has the theoretic advantage of delivering insulin in a more physiologic manner, because insulin undergoes a “first pass” through the liver, and the hyperinsulinemia that results from systemic drainage is avoided (Gaber et al, 1995). There is also an immunologic advantage of portal drainage that has been observed in several experimental studies, in which the delivery of foreign antigen via the portal system results in diminished immune responses. Despite these potential advantages, no demonstrable difference has been found in outcomes between human transplants drained by the portal vein and those drained by the systemic vein, and systemic venous drainage is how the majority of pancreas transplantations are performed.
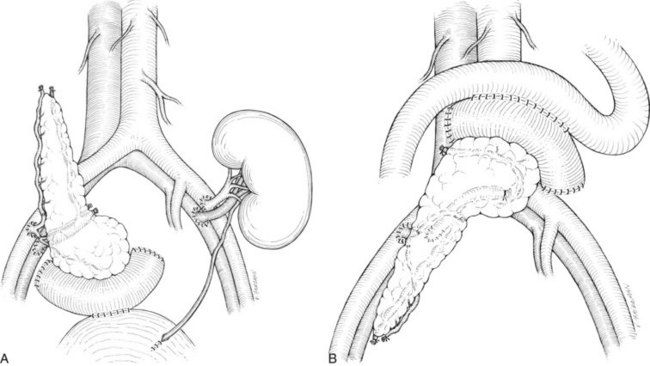
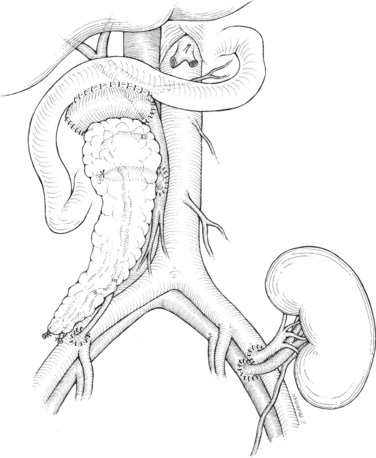
There are two common locations in the abdomen where the transplant is placed based on the type of venous drainage planned: either in the pelvis, most commonly the right side, for systemic venous drainage or in the midabdomen for portal venous drainage. When the graft is placed in the pelvis, the donor portal vein is anastomosed to the external iliac vein, the common iliac vein, or the inferior vena cava. In this pelvic position, the graft is oriented with the duodenum in an inferior position, if bladder drainage is planned (Fig. 101.1A), or with the duodenum in either the superior (Fig. 101.1B) or inferior position if enteric drainage is planned. Alternatively, for portal venous drainage, the pancreas is placed in the mid-abdomen below the transverse colon with the duodenum oriented superiorly. The portal vein of the pancreas is anastomosed to a major branch of the superior mesenteric vein, found in the small intestine mesentery, in an end-to-side fashion (Fig. 101.2). Enteric drainage for exocrine secretions must be used with the portal venous drainage technique. With either venous drainage technique, the donor arterial conduit to the pancreas graft is anastomosed in an end-to-side manner to the recipient common or external iliac artery.
Complications
The major complications following pancreas transplantation are often technical in nature. Pancreas graft thrombosis, arterial or venous, is more frequent after pancreas transplantation than after other solid-organ transplants, and the reported incidence is approximately 10%. Thrombosis usually occurs within the first week following transplantation and likely reflects the relatively low blood flow through the organ. In most instances of thrombosis, graft removal is necessary (Humar et al, 2000). Early pancreatitis occurs in 10% to 20% of cases and is largely a reflection of ischemic damage to the gland during preservation and reperfusion injury. Hyperamylasemia and graft edema are characteristic, and graft pancreatitis is usually treated nonsurgically with octreotide. Leakage at the site of pancreatic exocrine drainage is another early complication, with management often dictated by the method of drainage. Bladder-drained transplants with a small leak at the duodenocystostomy can often be managed by Foley catheter drainage of the bladder, allowing the site of leakage to heal over time. Enteric-drained transplants with a leak at the duodenojejunostomy will often result in peritonitis and usually require operative intervention to control the leak.
Results
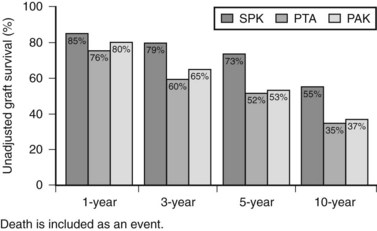
Patient and graft survival following pancreas transplantation have improved significantly in recent years. Depending upon the type of transplantation—SPK, PAK, or PTA—patient survival is approximately 96% to 98% at 1 year and 85% to 89% at 5 years after transplantation. Graft survival varies according to the type of transplantation performed (Fig. 101.3). For SPK recipients, 1-, 5-, and 10-year pancreas graft survival rates are 85%, 73%, and 55%, respectively. For PAK recipients, the respective rates are 80%, 53%, and 37%. Finally, for PTA recipients, rates of pancreas graft survival are 76%, 52%, and 35%, respectively (Axelrod et al, 2010).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree