Chapter 97E Liver transplantation for cholangiocarcinoma and other neoplastic diseases
Hilar Cholangiocarcinoma (See Chapter 50B)
CCA, the second most common primary malignant tumor of the liver, arises from the cholangiocytes of the intrahepatic and extrahepatic bile ducts. The incidence of this tumor has been estimated at 3000 to 5000 cases per year (de Groen et al, 1999; Olnes & Erlich, 2004), and the prevalence of intrahepatic disease appears to be on the rise (Shaib et al, 2004). CCA can arise within the liver, in the perihilar location, or along the extrahepatic bile duct (see Chapters 50A and 50B). CCA has three growth patterns: 1) mass-forming tumors are usually intrahepatic, 2) sclerosing tumors arise in the perihilar and extrahepatic bile ducts, and 3) polypoid tumors grow within the major intrahepatic and extrahepatic ducts. Surgical extirpation has been the standard treatment for all three tumor types.
The treatment of hilar CCA has been most troublesome because it is impossible to achieve a tumor-free (R0) margin of resection. Radical resection with partial hepatectomy was shown to improve survival (Launois et al, 1979) for patients with hilar CCA, but few patients come to medical attention with disease amenable to complete resection. Indeed, fewer than 30% of patients are candidates for resection at diagnosis because of either bilateral liver involvement, encasement of hilar vascular structures, involvement of sectoral bile ducts, and/or underlying liver disease, such as primary sclerosing cholangitis (PSC; see Chapter 41). Liver transplantation appears promising for the treatment of intrahepatic and hilar CCA, because the procedure affords a radical resection, is not limited by bilateral ductal or vascular involvement, and treats underlying liver disease.
Early Experience with Liver Transplantation
Unfortunately, early experiences with liver transplantation for the treatment of CCA were uniformly poor. Liver transplantation for both intrahepatic and hilar CCA was fraught with high recurrence rates and poor patient survival. The Cincinnati Tumor Registry reported a large multicenter analysis for patients transplanted from 1968 to 1997, wherein 1-, 3-, and 5-year patient survival rates were only 72%, 48%, and 23%, respectively (Meyer et al, 2000). The recurrence rate was 51% with a median time to recurrence of only 9.7 months. Local recurrence within the allograft was the most common initial site of recurrence (47%) followed by distant metastases to the lung (30%). Recurrence of tumor portended an extremely poor prognosis, with a median survival of only 2 months. Adjuvant therapy was not found to be beneficial, and no difference was reported in the survival rate of known tumors versus incidental tumors found at the time of orthotopic liver transplantation (OLT); results were poor for both intrahepatic and hilar tumors.
A multitude of retrospective studies have confirmed these findings. A Spanish multicenter study reported similar results for 59 patients who underwent OLT for CCA from 1988 to 2001 (Robles et al, 2004) and found 5-year survival was 30% with a 53% recurrence rate for 39 patients with hilar CCA. Results were equally poor for 23 patients with intrahepatic CCA, for which 5-year survival was 42%, and the recurrence rate was 35%. Similarly, a Scandinavian study reported a 5-year survival of 30% following OLT in a primary sclerosing cholangitis (PSC) population with early-stage CCA (Brandsaeter et al, 2004).
Several centers have reported their outcomes with incidental tumors discovered in patients undergoing transplantation for chronic liver disease. Ghali and colleagues (2005) reviewed the Canadian experience from 1996 through June 2003 and identified 10 cases, 8 arising in patients with PSC. Most of these tumors had favorable characteristics that included small size (<1 cm) and absence of perihepatic lymph node involvement; 90% were well differentiated, and 60% arose in the extrahepatic or hilar ducts. Despite the favorable characteristics, 3-year survival was only 30%. Only the University of California–Los Angeles has reported reasonable survival outcomes in incidental CCA detected in the explant after OLT (Goss et al, 1997). Ten patients with incidental CCA had a 5-year survival of 87%, which was comparable to PSC patients without CCA, although pathologic characteristics were not included in the paper. As with all other experiences, the 4 patients transplanted with known CCA had poor outcomes, and none were alive at 5 years.
A more radical approach with cluster abdominal transplantation reported by the University of Pittsburgh had equally poor results: a 3-year survival of 20% and a 57% recurrence rate (Alessiani et al, 1995). A similar experience was recently reported by Neuhaus’ team in Berlin (Seehofer et al, 2009). Sixteen patients with CCA were treated by combined liver transplantation and pancreatoduodenectomy (PD) between 1992 and 1998, and results were compared to those achieved for 8 patients who did not undergo PD, which at the time of liver transplantation was associated with significantly higher morbidity than transplantation alone. Long-term survival (>4 years) was achieved in only 3 lymph node–negative patients of 20 patients who survived the perioperative period. Neuhaus and colleagues concluded that “there is no good evidence that more radical resections alone are able to markedly improve long-term results.”
Neoadjuvant Therapy and Liver Transplantation
Despite the poor results with liver transplantation alone, some patients with favorable hilar CCA—that is, those with negative margins and no regional lymph node metastases—did benefit from transplantation (Shimoda et al, 2001). In addition, a small group of patients at the Mayo Clinic treated with primary radiotherapy and chemosensitization alone, without resection, had a 5-year survival of 22% (Foo et al, 1997). Based on the known palliative efficacy of radiotherapy for CCA—and knowledge that CCA resection failures are usually due to locoregional recurrence, rather than distant metastases (Jarnagin et al, 2003)—the transplant team at the University of Nebraska pioneered a strategy of high-dose neoadjuvant brachytherapy and chemotherapy followed by liver transplantation (Sudan et al, 2002) for patients with unresectable hilar CCA.
Mayo Clinic Experience
Inclusion and Exclusion Criteria
Criteria for protocol enrollment are designed to select those patients least likely to develop metastatic disease, most likely to respond to neoadjuvant therapy, and who have a high probability for survival after transplantation. Appropriate patients include those with early-stage hilar CCA determined to be unresectable or those who have underlying PSC, because CCA arising in the setting of PSC has a very poor natural history following standard resection (Rosen et al, 1991).
Staging Operation
Prior to 2003, 30% to 40% of patients had findings during the staging operation that precluded transplantation. EUS-guided aspiration of regional nodes (not the primary tumor) was implemented to exclude patients with lymph node metastases prior to initiation of neoadjuvant therapy. Initial findings from 47 patients identified eight (17%) with metastases (Gleeson et al, 2008). No morphologic features of the lymph nodes were found at EUS that predicted microscopic disease. Since routine use of EUS was implemented in 2003, the percentage of patients with a positive staging exploration has been reduced to 15%. EUS avoids the morbidity and mortality of high-dose neoadjuvant therapy and prevents an unnecessary operation for patients destined to fall out at operative staging.
Results
One hundred ninety-six patients were enrolled in the Mayo Clinic protocol from 1993 until April 10, 2010 (Fig. 97E.1). Sixteen patients died, became too debilitated for transplantation, or had disease progression prior to operative staging. Three patients underwent transplantation at other centers, and five were receiving neoadjuvant therapy and/or awaiting operative staging. One hundred seventy-two patients underwent operative staging, and 38 patients (22%) had findings precluding transplantation. After staging, 1 patient died from hepatic decompensation, 1 developed intrahepatic metastases, and 3 patients underwent transplantation at other centers. The remaining 126 patients underwent transplantation, 84 with deceased-donor grafts, 41 with living-donor grafts, and one with a domino familial amyloid donor graft.
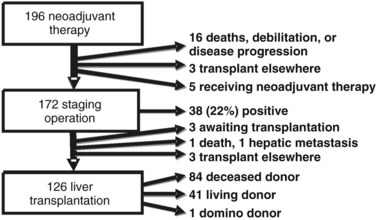
FIGURE 97E.1 Mayo Clinic neoadjuvant therapy and liver transplantation for hilar cholangiocarcinoma, 1993 through 2010.
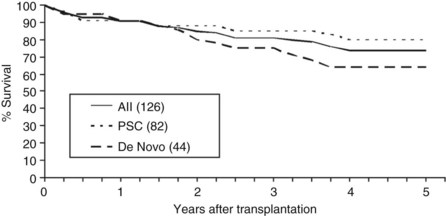
Overall patient survival from enrollment in the protocol (intention-to-treat analysis) was 56% ± 4% at 5 years after the start of neoadjuvant therapy (Fig. 97E.2), 63% ± 5% for patients with underlying PSC, and 44% ± 7% for patients with de novo CCA. Five-year survival after transplantation was 74% ± 5%; 80% ± 5% for patients with underlying PSC and 64% ± 8% for patients with de novo CCA (Fig. 97E.3). No difference in survival was reported among patients undergoing LDLT versus DDLT.
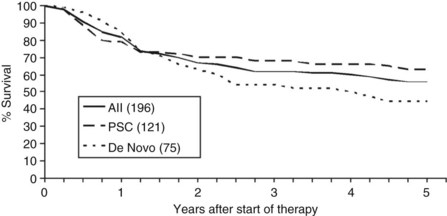
FIGURE 97E.2 Patient survival after start of neoadjuvant therapy. PSC, primary sclerosing cholangitis.
Recurrence and Prognostic Factors
Twenty-one of the 125 patients (17%) who underwent OLT developed recurrent disease at a mean of 25 months (range, 7 to 64 months) after transplantation, and 10 (48%) of the 21 recurrences were distant metastases. The initial sites for recurrent CCA are shown in Table 97E.1.
Table 97E.1 Cholangiocarcinoma Recurrence After Transplantation
| Site | Time (mo) |
|---|