Fig. 7.1
Age-standardized incidence ( blue + red columns) and mortality ( red columns) from gastric cancer in 2012 in men a and women b (GLOBOCAN 2012). Only countries with at least score E for availability of incidence data and score 4 for availability of mortality data were included. Some European data were not reported to avoid column superimposition
Large differences in incidence exist also within the same continent: for instance, within Europe, gastric cancer incidence varies four to fivefold, being particularly high in Eastern Countries (24.5 and 10.8 per 100,000 person-years in Russian men and women) and particularly low in Scandinavia (4.9 and 2.7 per 100,000 person-years in Swedish men and women). Likewise, in South America, incidence is much higher on the Pacific coast than in countries facing the Atlantic Ocean.
Gastric cancer is the third leading cause of cancer death worldwide. The pattern of mortality largely reflects the pattern of incidence, being the highest in Eastern Asia and the lowest in North America. At variance mortality is higher in China (25.5 and 10.7 per 100,000 person-years in men and women) than in South Korea (19.6 and 7.9 per 100,000 person-years), where incidence is nevertheless twofold higher. Indeed the ratio of deceased to incident cases varies largely worldwide, being as low as 34.4 % in South Korea, close to 50 % in Japan (48.5 %) and USA (55.6 %), 71.6 % in the European Union and 80.3 % in China in 2012. In most developing countries mortality rate approaches incidence rate. (Of note, to compare mortality and incidence within the same country, we did not use standardized rates that, using as reference the world population, would have attributed larger weights to incidence than to mortality. Direct standardization was instead mandatory when comparing incidence and mortality across different countries, as in Fig. 7.1.)
Over the last 30-year period, there has been a marked decline in age-standardized incidence and mortality from gastric cancer, which involved almost all populations: from 2000–2004 to 2005–2009 mortality from gastric cancer among men declined by 26 % in South Korea, by 15 % in Japan, by 17 % in the European Union and by 15 % in the USA [2]. Similar changes were observed in women.
A decrease in the rate of infection with Helicobacter pylori is the most important factor contributing to the reduced stomach cancer burden worldwide [3]. The decrease in incidence is partly counteracted by population ageing.
Clinico-Pathological Characteristics in Western Countries
The declining incidence in stomach cancer throughout the world is mostly attributed to a fall in incidence of distal, intestinal type tumours, which correlates with the decreasing prevalence of H. pylori infection [4]. As the large variability in gastric cancer incidence between high- and low-incidence countries mainly reflects a large discrepancy in new cases of intestinal distal cancer, there is currently a higher proportion of cardia, proximal and diffuse-type gastric cancer, especially signet ring cell (SRC) [5], in countries with lower incidence and mortality rates of stomach cancer.
Indeed in contrast to adenocarcinoma of the distal oesophagus, which has increased markedly, the literature on temporal trends of cardia and proximal gastric cancer is somewhat conflicting, with decreasing, stable and increasing incidence rates reported [6–10]. However, considering the steep declining of intestinal distal type, the proportions of cardia and proximal tumours have been increasing over the last decade in almost all populations [11–15].
A recent report [2] highlights that the contribution from the cardia as a proportion of the total gastric cancer incidence varies as a function of gastric cancer incidence, being the lowest in South Korea (5.8 % in men and 4.3 % in women) and the highest in Northern Europe (72 % in Finnish men and 44.5 % in British women). Indeed, cardia cancer exceeds non-cardia cancer in several male populations of Northern (Finland, Denmark, UK) and Central Europe (Belgium, Austria). Moreover, a proportion close to 50 % is observed in men of most Anglosaxon countries (USA, Canada, Australia, New Zealand).
At variance with intestinal-type gastric cancer, the incidence of diffuse gastric cancer, particularly the SRC type, has been increasing and nowadays represents a great proportion of stomach tumours in Western series. When analyzing the Surveillance Epidemiology and End Results (SEER) database using the Lauren classification, the intestinal histotype decreased by 52 % from 1973 through 2000 (on average 2.4 % per year), while the diffuse histotype increased by 441 % in the same time period (3.6 % per year). The highest rise was observed for the SRC type which increased by 6.5 % per year, i.e. by 998 % from 1973 to 2000 [5]. Thereafter, a decreasing trend has been found for absolute diffuse cases, which is probably related to changes in coding procedures [14], while the ratio of diffuse compared to intestinal histotype is still increasing, especially for non-cardia gastric cancer.
Different epidemiological trends strengthen the hypothesis that proximal intestinal, distal intestinal and diffuse subtypes of gastric cancer may be distinct diseases, related to different risk factors and thus characterized by different biological behaviour.
It is interesting to note that recent Western reports [16], including a GIRCG (Italian Research Group for Gastric Cancer) clinical study on temporal trends [10], show no improving or even worsening prognosis in recent years, despite the enhanced clinical, surgical and oncological quality. These findings may be due to the above-mentioned changes in clinico-pathological features of gastric cancer: distal intestinal tumours, which are declining in Western countries, present the most favourable prognosis. Accordingly, Dutch and French epidemiological studies reported a clear-cut increase in the frequency of gastric linitis plastica and metastatic forms [17, 18], while in an Italian study the rate of peritoneal recurrence showed a progressive increase after radical surgery, with respect to locoregional or hematogenous spread [10].
Western Surgical Approach: Historical Perspective
The large difference in gastric cancer incidence is one of the main reasons of the remarkable discrepancies in treatment strategies between Eastern and Western countries [20].
The extent of lymphadenectomy has been the most debated issue in gastric cancer surgery. Indeed, Japanese surgeons have routinely performed extended lymphadenectomies since decades, while Western surgeons preferred limited nodal dissections.
Western approach to advanced gastric cancer was largely influenced by the results of two randomized clinical trials (RCT) , the UK Medical Research Council and Dutch Gastric Cancer trials [21, 22], reporting that D2 provided no 5-year survival advantage with respect to D1. Of note, the two trials had been carried out by surgeons without previous training in extended lymphadenectomy , with a surgical volume of less than five procedures per year. The limited surgical experience yielded a very high postoperative mortality after D2 dissection (9.7 % in the Dutch trial and 13.5 % in the British trial), probably related to the high percentage of splenectomies (37 and 65 %, respectively) and pancreatectomies (30 and 56 %) [23].
A Cochrane review, published in 2003 and 2005 [24, 25], taking into account the results of the above mentioned Western RCT concluded that extended lymphadenectomy does not offer survival benefits. However, the authors stated that “their results could be confounded by surgical learning curve and poor surgical compliance”.
Despite of evidence-based indications, D2 lymphadenectomy has been routinely performed in the last two decades in high-volume Western centres. D2 lymphadenectomy is currently considered the standard of surgical treatment with curative intent by the German [26, 27] and British [28] national guidelines, the European Society for Medical Oncology (ESMO) guidelines [29], the joint ESMO—ESSO (European Society of Surgical Oncology—ESTRO (European Society of Radiotherapy and Oncology) guidelines [30] and the Italian Society of Surgery (SIC)-GIRGC guidelines [31]. At variance American NCCN guidelines recommend a D1+ or a modified D2 lymph node dissection [32].
Indexes of Surgical Quality in Gastric Cancer Surgery
In a previous study [33], we proposed as indexes of surgical quality the number of retrieved nodes, the percentage of splenectomy and distal pancreasectomy, the rate of postoperative complications and mortality. These indexes vary widely between the East and the West, probably as a function of surgeons’ experience.
Indeed, extended lymphadenectomies were associated with a high number of harvested lymph nodes (median 39–54) and with a low (0.8 %) or even absent postoperative mortality in Eastern trials [34, 35]. Conversely, in the Dutch and British clinical trials, D2 lymphadenectomy was associated with a lower number of retrieved lymph nodes and to a high postoperative mortality (10–13 %) [21, 22].
In Western observational studies, indexes of surgical quality were intermediate between Eastern and European trials. Indeed in a GIRCG series, D2 lymphadenectomy was safely performed with a median number of harvested nodes of 29 and an adequate lymphadenectomy (> 15 lymph nodes) achieved in 94 % of cases [33].
In the recent years, it has been acknowledged that the unsatisfactory short-term results of the British and Dutch trials could have been avoided with an adequate training of participating surgeons. Indeed a more recent Western trial [36] comparing D1 and D2 lymphadenectomy, preceded by a phase 2 trial and including only experienced surgeons, presented a mortality of 2.2 % after D2 dissections, further supporting that extended lymphadenectomy is a safe therapeutic option also in Western patients.
The quality of the surgical procedure surely affects short-term results, but can also influence long-term survival. In Fig. 7.2, long-term survival of the most important RCTs [21, 22, 37–39] dealing with the extension of lymphadenectomy is presented as a function of the number of retrieved lymph nodes. When the trials are separately considered, discordant trends are apparent (panel A). After excluding patients dying in-hospital (panel B), an improvement in survival becomes clearly apparent when the more extended procedure allows to retrieve > 10 lymph nodes than the other procedure. No survival advantage is observed when > 50 nodes are detected also with the more limited procedure. If the five trials are considered together, 5-year overall survival seems to increase as a function of retrieved nodes reaching a plateau around 25–30 retrieved nodes. In addition, in the Italian trial, the high proportion (33 %) of early gastric cancer (pT1) can partly explain the lack of benefit after the D2 procedure [40].
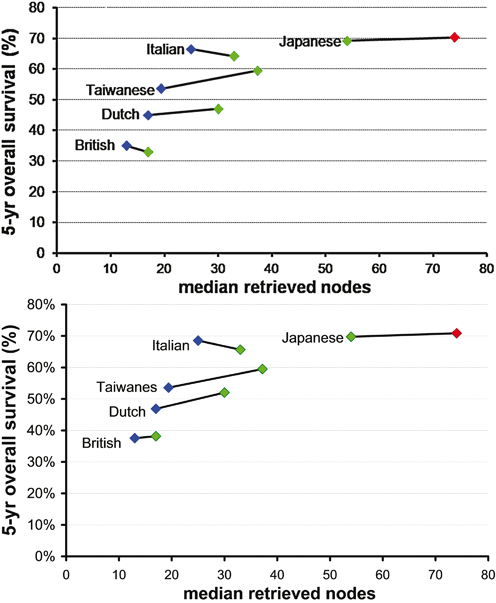

Fig. 7.2
Five-year overall survival as a function of median retrieved nodes in five clinical trials. Four trials (British, Dutch, Taiwanese, Italian) compared D1 ( blue diamonds) and D2 ( green diamonds), while the Japanese trial compared D2 and D2 + PAND ( red diamond). Panel A: whole series. Panel B: Patients dying in the postoperative period were excluded. PAND para-aortic nodal dissection
This new perspective on the results of the most recent trials, based on the number of retrieved nodes rather than the planned extension of lymphadenectomy, further supports the central role of D2 lymphadenectomy in gastric cancer surgery, when performed by experienced surgeons.
Some authors [41] pointed out that in the USA, the low volume of gastrectomies is the main obstacle in implementing extended lymphadenectomies, indeed in the USA, 80 % of medicare patients undergo gastrectomy in centres performing less than 20 procedures per year [42]. That is the reason why NCCN Guidelines still include D1 resection as an acceptable procedure, but request a minimum of 15 dissected lymph nodes [32].
In Europe, many efforts are ongoing to increase the number of D2 dissections according to the above-mentioned guidelines, and centralization seems to be crucial in this context. Indeed in the Netherlands, survival of gastric cancer patients significantly improved after the implementation of the Dutch D1-D2 Gastric Cancer trial, which involved substantial standardization and training [43]. In Denmark, 30-day hospital mortality has decreased from 8.2 to 2.4 % after centralization of gastric cancer surgery and implementation of national clinical guidelines while the proportion of patients with at least 15 lymph nodes removed has increased from 19 to 76 % [44]. Centralization of gastric cancer surgery and/or audits for gastric cancer are currently implemented in UK, Sweden, Finland and the Netherlands [45, 46].
Survival Outcomes: West Still Differs from the East
As reported above, specialized high-volume Western centres currently provide high quality standardized surgical management of gastric cancer patients [33]. In GIRCG centres, [47] survival rates are very high and similar to Eastern series [48, 49], particularly in stages I and II, although in more advanced stages (IIIB and IIIC) lower survival rates than Eastern series are observed. However, even if there are some differences in series characteristics and survival end-points in papers reporting survival according to 7° TNM classification [49–51], long-term outcomes reported in other Western series [50– 51] are remarkably worse than in Eastern series at each stage (Table 7.1).
Table 7.1
Five-year survival by stages in Eastern and high-volume Western series reported in the literature according to 7th TNM
Ia (%) | Ib (%) | IIa (%) | IIb (%) | IIIa (%) | IIIb (%) | IIIc (%) | |
|---|---|---|---|---|---|---|---|
Kikuchi et al. [49] | – | 94.30 | 84.80 | 71.30 | 64.8 | 48 | 23.1 |
Ahn et al. [48] | 95.1 | 88.40 | 84 | 71.70 | 58.4 | 41.3 | 26.1 |
Marrelli et al. (GIRCG) [47] | 97 | 89 | 86 | 69.00 | 59 | 35 | 11 |
et al. [50] | 81 | 58.00 | 55 | 35.00 | 32 | 13 | 10 |
Warneke et al. [51] | 64 | 41 | 34 | 21 | 16 | 6 | 5 |
Strong et al. [52] compared two high-volumes centres in USA and South Korea using an internationally validated nomogram , and found better survival for Korean patients. The reasons why survival in gastric cancer patients remains worse in the West despite improvements in surgical quality are not fully understood yet. Tentative explanations could be the following:
Differences in clinico-pathological features of gastric cancer in Eastern and Western countries could partially be responsible of the different outcomes. As already discussed, in low-incidence countries proximal and diffuse types are currently the most frequent subtypes of gastric cancer.
In many series [53–55], location of tumour in the proximal third has been shown to be an independent negative prognostic factor. The worse prognosis of proximal tumours has been explained by the more advanced stage, the larger size and the poorly differentiated histology, which are typical of this subtype of gastric cancer [56].
The real association between hystotype and survival of patients with gastric cancer is controversial. Indeed the prognostic significance of histology usually disappears after controlling for pTNM. It should be reminded, however, that Lauren diffuse cancers are more prone to give lymph node metastases; hence, stating that Lauren histotype is not an independent prognostic factor when adjusting for N status is not correct, as N status is not a confounder but rather an intermediate step in the causal pathway. In the Verona series, Lauren histology was an independent prognostic factor when N classification was based on site (TNM 1987) but not when based on number of positive nodes (TNM 1997) [57].
In particular, as regards SRC histology, the prognostic significance seems to be strictly “dependent on stage” according to a recent American study [58]. Indeed SRC adenocarcinomas show a more aggressive behaviour and lower disease-related survival compared to non-SRC tumours only in advanced stages. It has been hypothesized that “driver mutations controlling the metastatic potential of SRC may occur late in the course of disease, rendering SRC tumours which are relatively indolent in the early stages, highly aggressive in more advanced stages” [58].
The low survival rate reported for this subtype of gastric cancer in advanced stages is mainly related to the high potential of peritoneal dissemination when tumours involve serosa layer. Indeed as shown in Table 7.2, the largest survival gap between Eastern and high-volume Western series emerges for serosa arising tumours probably due to the higher percentage of diffuse-SRC type.
Thus, the prevalence of more aggressive cancers could explain the worse Western survival in advanced stages. Unfortunately, only few studies comparing Eastern and Western series according to gastric cancer subtypes are available yet. However, recent reports [59] suggested that when gastric cancer series with homogeneous clinico-pathological features are compared, survival between Eastern and Western patients is more similar than previously believed.
Disparities in tumour biology and patients’ ethnicity may be additional factors responsible of different prognoses observed across the world regions.
Several recent studies reported a better outcome in Asian Americans than in other ethnicities, and these differences persisted when results were adjusted for several tumour or treatment-related factors [60, 61].
A recent Italian study documented different prognoses in patients with gastric cancer coming from different risk areas of Italy treated at the same centre with a similar surgical approach and staged in the same way [62]. Patients coming from Southern Italy, where the incidence of gastric cancer is very low, showed a worse outcome when compared with patients coming from Tuscany, a high-risk area. These results were confirmed even considering surgical and pathological factors previously included in an Italian prognostic score, thus suggesting biological differences between the groups [63].
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







