Fig. 1 a–f
Complicated Stanford type B aortic dissection with renal malperfusion. a Computed tomography (CT) demonstrating primary entry tear of type B aortic dissection. b CT demonstrating compression of true lumen of the aorta with left renal ischemia. c Aortic angiogram showing true and false lumen of type B dissection. d Aortography showing compression of true lumen with malperfusion of the renal arteries (“floating visceral sign”). e CT after stent-graft implantation and closure of primary entry tear with false lumen collapse. f Aortography post stent-graft implantation demonstrating spontaneous revascularization of visceral arteries
In case of a static compression due to a side-branch dissection, stent placement in the true lumen of the organ artery will cause reconstitution of organ perfusion.
In case of organ perfusion through the false lumen, balloon fenestration of the intimal flap will reestablish flow into the malperfused territory.
Chronic Arterial Occlusive Disease
In young patients, causes of chronic arterial occlusion may be:
Fibromuscular disease (FMD)
Takayasu arteritis
Neurofibromatosis Recklinghausen.
In elderly patients, the primary cause of chronic arterial occlusive disease is arteriosclerosis.
Mesenteric Artery Stenosis
Between the three large mesenteric arteries (celiac artery, SMA, IMA), many collateral pathways exist:
Pancreaticoduodenal arteries
Arc of Buehler between celiac artery and SMA
Arc of Riolan
Marginal artery of Drummond between SMA and IMA.
Usually, an obstruction of more than one mesenteric artery is necessary to cause ischemic symptoms. The typical clinical symptom is “abdominal angina,” with:
Abdominal pain (94%)
Postprandial cramps (86%)
Weight loss (74%)
Abdominal bruit (70%)
Diarrhea.
The primary diagnosis is made by CT angiography, magnetic resonance angiography (MRA), or intra- arterial catheter angiography of the abdominal aorta in a lateral projection. Interventional treatment is percutaneous transluminal angioplasty (PTA) with or without secondary stent placement of the obstructed arteries.
Celiac Trunk Stenosis
Chronic obstruction may remain asymptomatic because of the collateral pathways through gastroduodenal and pancreatic arteries from the SMA. Causes are arteriosclerotic plaque, compression by the arcuate ligament, and carcinoma of the pancreas.
Superior Mesenteric Artery Stenosis
Postprandial abdominal pain, called “abdominal angina,” is the leading symptom. Causes for SMA obstruction are arteriosclerosis, FMD, Takayasu arteritis, pancreatic carcinoma, and chronic pancreatitis.
Inferior Mesenteric Artery Stenosis
Obstruction of the IMA is most commonly observed in patients with advanced atheromatosis or partially thrombosed abdominal aortic aneurysm. Due to the collateral circulation through the arc of Riolan and the marginal artery, IMA obstruction normally remains asymptomatic.
Renal Artery Stenosis
Renal artery stenosis (RAS) may cause hypertension and/or renal insufficiency. Acute onset of clinical symptoms and repeated flash pulmonary edema are suggestive for RAS. Etiology can be
Arteriosclerosis in 65–75%
Patients >50 years
Male >female
Proximal 2 cm of renal artery
Atherosclerotic changes of aorta
Bilateral in 30%.
FMD in 20–30%
Patients <50 years
Female:male ratio 5:1
Middle to distal renal artery, including branches
Bilateral involvement in 50–70%
“String of beads” appearance, aneurysms, dissections
No aortic disease.
Takayasu arteritis
Midaortic syndrome
Morbus Recklinghausen
Postradiation therapy.
RAS Diagnosis
The most appropriate algorithm for diagnosing RAS is not yet established.
Color Duplex Ultrasound
Color duplex ultrasound (US) is a noninvasive test but is a complex procedure that requires operator experience. An increased peak systolic velocity of >250 cm/s, a renal-to-aortic ratio of peak systolic velocity of >3.5, intrastenotic turbulence, and a flattened pulse wave in the periphery (pulsus tardus) are diagnostic criteria for RAS. The sensitivity of color duplex sonography for detecting RAS >70% is 72–92%. Color duplex US with an angiotensin- converting enzyme (ACE) inhibitor provides a positive predictive value (PPV) of 67–95% for cure or improvement after revascularization.
Nuclear Scan
A nuclear scan [renal scintigraphy with technetium-99m mercaptoacetyltriglycine (99mTc-MAG-3) or technetium- 99m diethylenetriaminepentaacetic acid (99mTc-DTPA)] with an angiotensin-converting enzyme (ACE) inhibitor (captopril 25 mg) shows delayed tracer washout within the poststenotic kidney. However, in bilateral disease and in chronic ischemic nephropathy, tracer lateralization is less evident. In a selected population with a clinically high risk for RAS, sensitivity for detection of a unilateral RAS >70% is 51–96% (mean 82%). Its PPV, with improvement of hypertension after revascularization, is 51– 100% (mean 85%). However, scintigraphy is much less sensitive in unselected patients, bilateral disease, impaired renal function, urinary obstruction, and chronic ACE inhibitor intake.
Newer Tests
Newer tests are gadolinium (Gd)-enhanced MRA and spiral CT angiography (CTA).
Gd-Enhanced MRA
For state-of-art MRA, high-field-strength systems with high-performance gradients are necessary for breath-hold 3D T1-weighted spoiled-gradient-echo imaging with short TR and TE. Intravenous administration of Gd contrast material (0.1 mmol/kg; flow rate 2 ml/s), a central k-space readout, and background subtraction are additional techniques to improve signal-to-noise ratio (SNR) and spatial resolution. Sensitivity for detecting RAS >50% is >95% with MRA (Fig. 2). The main limitations of renal MRA are evaluation of small, accessory renal arteries and branch vessels, presence of stents, and a tendency to overestimate moderate stenoses.
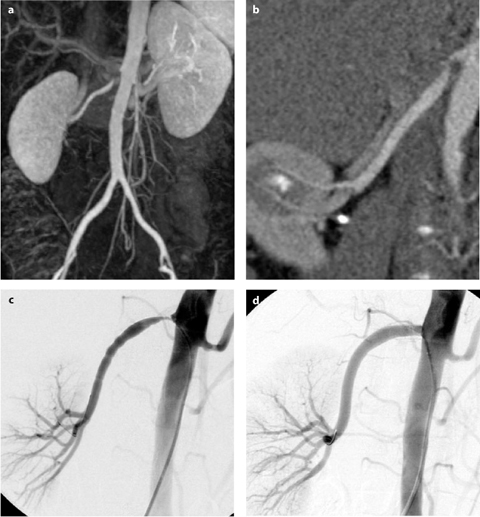

Fig. 2 a–d
Imaging and intervention in a patient with hypertension due to renal artery stenosis. a Magnetic resonance angiography (MRA) showing stenosis of right renal artery. b Computed tomography angiography (CTA) showing stenosis of right renal artery. c Arteriography showing stenosis of right renal artery. d Arteriography of right renal artery after stent placement
CTA
CTA of the renal arteries has a >95% sensitivity to detect RAS and accessory renal arteries. For high-quality opacification of renal arteries and to avoid renal vein overlap, accurate bolus planning is mandatory: density measurement during bolus rise, flow 4 ml/s, total volume 80–120 ml; multidetector (MD) scanners need less contrast. A short breath-hold acquisition, collimation (1–2 mm), pitch (1.5–6 depending on single or MD technology), reconstruction overlap (0.5–0.75) are important parameters for spatial resolution. Curved planar reconstruction (CPR, most useful for stents), volume rendering, and maximum intensity projection (MIP) are used for 3D imaging (Fig. 2).
Intra-Arterial Catheter Arteriography
Intra-arterial catheter arteriography together with pressure-gradient measurement is still the “gold standard“ for RAS evaluation.
RAS Revascularization
The revascularization technique of choice is renal PTA with or without stent placement (Fig. 2). Aortorenal bypass surgery is indicated only if PTA fails. In a recently published meta-analysis, stent placement proved to be technically superior and clinically comparable with renal PTA alone. The technical success rate of stent vs. PTA was 98% vs. 77%, and the restenosis rate was 17% vs. 26%, respectively (p<0.001). In hypertension, the cure rate of PTA vs. stent was 10% vs. 20% and improvement rate was 53% vs. 49%, respectively. In renal insufficiency, the improvement rate was 38% vs. 30% and stabilization 41% vs. 38%, respectively. Complication rate was 11–13% [95% confidence interval (CI) 6–19] and inhospital mortality rate 1%. In a randomized study comparing stents vs. PTA in ostial stenoses, the technical success rate was 88% vs. 57% and the 6-month primary patency rate 75% vs. 29%, respectively. Surprisingly, randomized trials comparing the effect of PTA and drug therapy on renal hypertension did not reveal a significant benefit of PTA and stenting over continuous drug therapy. However, in a Dutch study, PTA patients required only 2.1 vs. 3.2 daily drug doses (p<0.001); 22/53 patients in the drug group had to be switched to the PTA group because of persistent hypertension or deterioration of renal function.
Aneurysms
Abdominal Aortic Aneurysm (AAA)
The incidence of AAA in European adults ⩾60 years is 2.5%. Up to 10% of patients with symptomatic peripheral arterial disease (PAD) die from aneurysm rupture. Standard treatment is open surgery. However, endovascular implantation of stent-grafts is a new, emerging technique that may replace open surgery. Since the first clinical implant of a tube stent-graft in 1990, many different designs have been developed and tested in feasibility studies. Most recently, randomized studies [Endovascular Aneurysm Repair (EVAR) 1 trial; Dutch Randomized Endovascular Aneurysm Repair (DREAM) trial] compared results of open vs. endovascular repair. In the EVAR trial, the 30-day mortality in the EVAR group was 1.7% (9/531) vs. 4.7% (24/516) in the open repair group (p=0.009). Four years after randomization, all-cause mortality was similar between groups (~28%; p=0.46), although there was a persistent reduction in aneurysm-related deaths in the EVAR group (4% vs 7%; p=0.04). Meanwhile, 60–80% of patients are treated by EVAR with stent-grafts.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








