Fig. 1 a–d
A patient with known hepatocellular carcinoma (HCC) previously treated with radiofrequency ablation presented with rising alpha-1-fetoprotein and carbohydrate antigen (CA) 19-9 levels. The presence of disease was suspected, and the patient underwent pre-operative positron emission tomography computed tomography (PET/CT) restaging with double-tracer modality. a 18F-fluorodeoxyglucose (FDG)-PET maximum intensity projection (MIP), as with b axial fused FDG-PET/CT scan, demonstrated no evidence of abnormality. c Conversely, 11 C-choline PET/CT MIP, as with the d axial fused choline PET/CT scan, showed a metabolically active tumor [maximum standard uptake value (SUVmax) 10.7] on liver segment V. Moderately differentiated HCC was confirmed histologically
18F-fluorothymidine is a marker of tumor proliferation and it was suggested, by a small sample size, that the level of its uptake has a prognostic value with reduced overall survival in lesions with a high uptake [4].
Biliary System Malignancy
Primary neoplasms of the biliary system are a heterogeneous group mostly composed of GBC and CCA.
Gallbladder Carcinoma
GBC is the fifth most common cancer of the gastrointestinal tract, with approximately 6,000 new cases each year. Only 10% of cancers are localized to the gallbladder upon detection. Hence, despite the poor ability of US and CT scanning to distinguish malignant from benign gallbladder disease, these studies are most commonly used to detect GBC. Despite preoperative assessment with traditional cross-sectional imaging modalities, up to a third of patients with primary biliary malignancy still undergo unnecessary surgery. The main contraindications to resectability found in surgery are occult peritoneal and liver metastases and, less commonly, vascular and lymph node invasion.
By contributing additional functional information, and when used in the correct context, FDG-PET/CT can allow earlier detection of tumors, identification of occult metastatic disease, characterization of equivocal lesions, assessment of therapeutic response, and globally more accurate staging for potential resection [3]. Some studies suggest that PET has a high sensitivity for detecting GBC (75–100%); however, several studies show poor sensitivity of FDG-PET/CT for detecting regional lymph node metastasis in GBC [14].
GBC is associated with a rate of peritoneal metastasis as high as 30–75 %, and the risk of metastasis strongly correlates with the presenting T stage. False-negative results can be related to mucinous adenocarcinoma; falsepositive results can take place in patients with flogistic or granulomatous reactions and in cases of adenomyomatosis [1].
Cholangiocarcinoma
CCA is a rare tumor arising from the epithelium of the intrahepatic or extrahepatic bile ducts, represents about 3% of all gastrointestinal malignancies, and is the second most common hepatic malignancy after HCC. Only a minority of patients who present with CCA have known risk factors, such as chronic biliary inflammation, cholestasis, and/or congenital abnormalities.
Despite advances in diagnostic techniques and new therapeutic strategies, a 5-year survival for CCA continues to be <5%. This is mainly attributed to the delayed diagnosis, because the tumor remains silent until it is advanced and obstructs the bile duct. More than 90% of CCA are well- to moderately differentiated adenocarcinomas with a tendency to develop desmoplastic reaction and early perineural invasion. Diagnosis and accurate staging are improved with better imaging and advanced cytologic techniques [15].
CCAs are classified according to their anatomic location as: intrahepatic (ICCA), perihilar, or distal extrahepatic (ECCA). Perihilar CCAs, first described as a separate entity by Klatskin in 1965 [16], represent 60–70% of all CCAs, whereas ICCA represents 5–10% and ECCA 20–30%.
According to the morphologic classification system proposed by the Liver Cancer Study Group of Japan, CCA is classified into mass-forming, periductal infiltrating, and intraductal growth types [17].
Carbohydrate antigen (CA) 19-9 and carcinoembryonic antigen (CEA) are the most commonly used tumors marker, and MR-cholangiopancreatography is the best available imaging modality for CCA. Meticulous interpretation of all available clinical and radiological data is recommended to determine resectability and avoid unnecessary interventions. Memorial Sloan Kettering Cancer Center has shown that FDG-PET/CT had an overall sensitivity of 78% for identifying the primary tumor for CCA, which changed manageability in nearly a quarter of all patients [18].
Histopathology of the tumor is very important; in fact, PET has significant accuracy in diagnosing nodular tumor subtypes but may be less sensitive and produce false-negative scans due to poor FDG uptake in infiltrative and mucinous subtypes [19]. Additionally, PET/CT is vulnerable to diagnostic errors when biliary stents or postendoscopic cholangitis are present, as well as in the setting of chronic biliary inflammatory conditions such as primary sclerosing cholangitis, common in CCA patients. Regarding N staging, the predominance of lymph node involvement is around 45% for all CCA, with distal ECCA having the highest incidence of nodal metastases. It is difficult to detect microscopic lymph node metastases by PET/CT, and in extrahepatic CCA, difficulties can be encountered in distinguishing between extrahepatic parts of the tumor itself and FDG accumulation in perihilar lymph nodes. Presence of distant metastases (e.g., lung, bone, peritoneal, distant lymph nodes) is seen in 30% of patients at the time of diagnosis and connected with survival of only a few months. PET/CT has a sensitivity of 94–100% in detecting distal metastasis (Fig. 2). Additionally, 18F-FDGPET/ CT appears to be an encouraging method in postoperative monitoring of CCA recurrence. A small series by Chikamoto et al. [20] found that PET/CT had a sensitivity of 80%; in these cases, conventional imaging is limited because of difficulties in differentiating tumor tissue and postoperative changes [21–23].
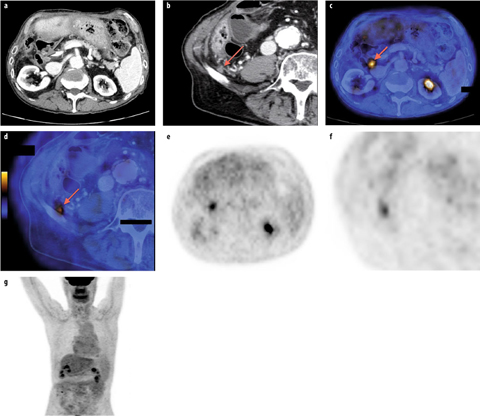

Fig. 2 a–g
Patient with known cholangiocarcinoma (CCA), previously treated with neoadjuvant chemotherapy and subsequently with surgery, started the radiotherapy for R1 margins of resection, subsequently interrupted for the appearance of hepatic abscesses that were surgically treated. Axial contrast-enhanced computed tomography (ce-CT) showed two doubtful lesions localized a in the precaval region at the level of the middle pole of the right kidney (arrow) and b in the posterior portion of the right iliac fossa immediately adjacent to the iliac bone (arrow). c, d Axial fused 18F-fluorodeoxyglucose positron emission tomography (FDG-PET)/CT images (arrows), as with e, f axial FDG-PET images, demonstrated two metabolically active findings corresponding to the ce-CT-detected lesions, suggestive for neoplastic foci. g No other pathologic uptake was demonstrated on FDG-PET maximum intensity projection image. Distant CCA metastases were confirmed surgically and pathologically
Pancreatic Malignancy
Pancreatic Ductal Adenocarcinoma
Pancreatic ductal adenocarcinoma (PA) is the fourth leading cause of cancer-related death, with a 5-year survival rate <5% after initial diagnosis. PA is relatively aggressive, and even when <2 cm can present early infiltration of the retroperitoneum and surrounding anatomic structures, including nerves and vessels. Serum CA 19-9 and CEA are detectable in ∼75% of patients with PA, and surgery remains the only curative treatment for locally resectable and nonmetastatic pancreatic cancer. In fact, <10–20% of PA is considered surgically resectable.
US, endoscopic retrograde cholangiopancreatography (ERCP), ce-CT, MRI, MR cholangiopancreatography (MRCP), and FDG-PET/CT are widely accepted imaging modalities accessible for PA diagnosis and staging. Unquestionably, PET/CT is valuable in PA management. In particular the potential indications for FDG-PET/CT include imaging-guided biopsy planning in cases with nondiagnostic FNA findings in patients with suspected pancreatic cancer, equivocal CT findings, tumor staging, depicting tumor recurrence, and monitoring response to therapy. Furthermore, it has proved useful in distinguishing postoperative fibrosis from recurrence. Actually, CT is limited in depicting small tumors (sensitivity 83% for lesions <2 cm) and isoattenuating lesions. About 10% of PA and pancreatic metastases are isoattenuating at ce-CT and, consequently, are not observed, even when >2 cm; differentiating mass-forming pancreatitis (MFP) from PA is commonly demanding. Both conditions are characterized by extensive fibrosis, with overlapping morphologic imaging findings. As PA may cause chronic obstructive inflammatory changes and MFP is associated with an increased risk for adenocarcinoma, the cross-sectional radiological detection remains challenging [24]. The reported sensitivity and specificity of FDG-PET/CT in assessing primary PA are 46–71% and 63–100%, respectively.
Staging pancreatic cancer is determined by local and distant spread of disease. Spread to lymph nodes is common and tends toward poor outcome. There are some limitations to current CT criteria for assessing lymph node involvement on the basis of size (>1 cm in the short axis) and in differentiating between benign and malignant lymph nodes. In N staging, some studies report PET/CT sensitivity and specificity ranging from 30% to 49% and 63% to 93%, respectively. The poor performance by FDGPET may be determined by the small tumor burden in metastatic lymph nodes and intense photon scatter from the primary tumor (known as the penumbra effect). Fused PET/CT may enhance the specificity of nodal staging compared with CT alone and consequently facilitate identification of metastatic deposits in lymph nodes that manifest as nonspecific or borderline enlargement at CT.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








