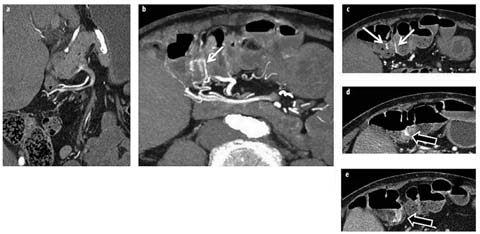
Fig. 1 a–d
a Crohn’s patient with ileitis (not shown) and normal feathery jejunal folds (arrow). b Crohn’s patient with jejunal inflammation manifested by asymmetric mural stratification, wall thickening, and hyperenhancement (arrows). c, d Celiac disease manifested by longsegment intramural fat in the duodenum and jejunum (c, white arrows), jejunal-fold atrophy (d, white arrow), and increased number of ileal folds (c, d black arrows)
Imaging of Inflammation
CT enterography is widely available as a cross-sectional imaging technique for adult patients with known or suspected Crohn’s disease and is recommended by the American College of Radiology (ACR) as one of the most appropriate tests for this indication. Additionally, even in centers where MR enterography is widely available to outpatients, patient volumes and symptomatic presentations to the emergency room (ER) will certainly necessitate CT imaging in patients with known Crohn’s disease. Initial cross-sectional studies necessarily focused on the ability of CT enterography to identify Crohn’s disease in the terminal ileum. Mural inflammation in Crohn’s disease is identified by the combination of segmental mural hyperenhancement and wall thickening, which is often asymmetric and accompanied by mesenteric border fibrofatty proliferation and comb sign. However, the dichotomous decision (Crohn’s present vs. absent) is no longer adequate for modern Crohn’s management. The length and severity of inflamed segments should be described, in addition to their multifocality. Interpretation should also include evaluation of potential strictures (i.e., luminal narrowing with upstream dilation), penetrating and obstructing complications, the colorectum and anus, mesenteric vessels, and other extraenteric findings [11]. When fistulae are seen, they should be categorized as simple or complex, with additional description of inflammatory masses and abscesses also given (whether amenable to percutaneous drainage or not). Comparison with prior studies should be performed to determine therapeutic response and potential bowel-wall remodeling [12].
Radiologists should be aware of emerging concepts in Crohn’s disease imaging that emphasize the complementarity of optical techniques, which evaluate only the mucosa, and cross-sectional techniques that visualize the small bowel wall. Whereas optical and cross-sectional imaging findings are often synchronous, up to 50% of Crohn’s patients with normal-appearing mucosa at ileoscopy will have unequivocal small bowel inflammation at CT enterography [13]. Furthermore, combining endoscopic and CT enterography findings leads to a greater correlation with serum markers of inflammation than ileocolonoscopy alone [14]. Whereas it has long been known that there is poor correlation between endoscopic and biologic markers of Crohn’s activity and patient symptoms [15], recent therapeutic trials highlight the link between intestinal healing and decreasing rates of hospitalization and surgery [16]. Thus, clinical decision making increasingly relies upon objective measures rather than protean patient symptoms for disease management. Clinical decisions often involve changing medical therapy, and importantly for quiescent disease, deciding whether current regimens should continue to treat obvious inflammation. In one prospective study of >250 patients with known or suspected Crohn’s disease in which gastroenterologists decided on potential management before and after CT enterography, management changes were altered by CT enterography results in 51% of patients overall [17]. CT enterography changed management decisions in nearly a quarter by excluding active inflammation, with medication changes occurring in approximately one quarter, and surgical plans being altered in 5–8%.
Segmental mural hyperenhancement and wall thickening are key findings in identifying Crohn’s-related inflammation but are not specific to Crohn’s disease. As mentioned, asymmetric and patchy hyperenhancement and wall thickening are pathognomonic for Crohn’s disease, particularly in conjunction with fibrofatty proliferation and the comb sign [18]. Other types of inflammation that may mimic Crohn’s disease can include ulcerative jejunitis from celiac disease, angioedema from angiotensinconverting enzyme (ACE) inhibitors, infectious and radiation enteritis, and tuberculous ileocolitis. Celiac disease will often be accompanied by villous atrophy in the jejunum and fold-reversal pattern in the small bowel, but will demonstrate symmetric wall thickening and hyperenhancement when ulcerative jejunitis is present, and involve long bowel segments (Fig. 1). Angioedema from ACE inhibitors will present with symptomatic abdominal pain in patients with a suggestive history. Radiation enteritis often presents with obstructive symptoms in patients who have undergone radiation therapy for cervical cancer. Primary small bowel lymphoma and carcinoid tumors can sometimes be mistaken for Crohn’s disease, and small bowel carcinoid tumors and adenocarcinomas can arise in Crohn’s strictures themselves. When mass-like findings at potential Crohn’s-related stricture locations are worrisome (for example, in the absence of hyperenhancement as may occur in lymphoma or with suspicious lymphadenopathy), further investigation with laparoscopy or balloon-assisted endoscopy may be warranted.
Cross-sectional imaging in Crohn’s disease is often used to monitor therapeutic response, as biologic agents have significant benefits but are also accompanied by risk and expense. In asymptomatic patients, MR enterography or ultrasound (US) is likely more appropriate for assessing therapeutic response [19]. Because the natural history of Crohn’s disease includes the development of penetrating and stricturing disease over time, with approximately 80% of patients undergoing surgery over a 20-year time period [20], symptomatic presentation to the ER will occur, and CT is the modality of choice in the emergent setting. Radiology departments should be prepared to perform CT enterography in Crohn’s patients, without enteric preparation, with low dose techniques. In the ER setting, abdominal CT results in a change in management in 81% of Crohn’s disease patients and 69% of ulcerative colitis patients, emphasizing a positive benefitto-risk ratio for CT imaging in patients with inflammatory bowel disease (IBD) [21].
Obscure GI Bleeding
Obscure GI bleeding (OGIB) is recurrent or persistent GI bleeding in which the small bowel is the likely source of GI blood loss because of prior negative upper and lower endoscopy. OGIB is characterized as “overt” when there is visible evidence of bleeding, such as melena, hematemesis, or hematochezia, or as “occult,” such as in the setting of iron-deficiency anemia or positive fecal occult blood test (FOBT). Multiphase CT enterography is an outpatient test that distends the small bowel lumen and requires a multiphase technique to evaluate for small amounts of active small bowel bleeding and identify and characterize bleeding masses, with imaging findings often suggesting the etiology of small bowel blood loss (vascular mass, tumor, inflammation, other).
Multiphase CT enterography is not used to search for acute, massive GI bleeding in the ER setting. No enteric contrast is used, and the goal of the examination is to identify the presence and site of active bleeding for treatment, or for selecting appropriate angiographic or endoscopic therapy [22]. Imaging in massive GI hemorrhage is not designed to identify or suggest bleeding etiology.
Whereas capsule endoscopy is now the first imaging test performed at many institutions in patients with OGIB, there is increasing recognition for the role of multiphase CT enterography in this challenging group of patients. CT enterography was included in the 2011 American Society for Gastrointestinal Endoscopy (ASGE) guidelines for OGIB [23], the principal reason being to identify mass lesions, which can be difficult at capsule endoscopy, as many small bowel tumors arise within the gut wall rather than the mucosa. Compared with balloonassisted endoscopy and CT enterography, capsule endoscopy identifies about one third of such tumors. In one prospective trial, significantly more small bowel bleeding sources were identified at multiphase CT enterography than at capsule endoscopy, principally owing to the identification of small bowel masses [7]. Studies suggest that the principal benefit of CT enterography is in patients with overt-type OGIB and those with nondiagnostic capsule endoscopy findings. The diagnostic yield of CT enterography in occult obscure GI bleeding is low. Whereas many capsule endoscopy studies focused on diagnostic yield, there is a large overlap in the prevalence of angioectasias, ulcers, and erosions in patients with OGIB and healthy volunteers.
Interpretation of multiphase CT enterography (MpCTE) data sets can be challenging owing to the large number of images with multiple phases and planes, and with inconsistent quality in the preceding endoscopic examinations. Colorectal neoplasia and vascular malformations are commonly missed at colonoscopy. Cecal and rectal arteriovenous malformations (AVMs) and varices are commonly detected at MpCTE. On this basis, interpretation is normally begun with identifying the right colic artery and vein, which is followed to the cecum, examining for enlargement and contrast within the right colic vein that may indicate a cecal AVM. To make the diagnosis of cecal AVM, this vascular shunting must be accompanied by focal (usually serpiginous), vascular wall enhancement that represents the AVM itself (Fig. 2). In a similar fashion, the superior hemorrhoidal artery is visually followed to the paired rectal vessels and rectum, where rectal vascular malformations are also detected. Colorectal masses are often best appreciated on entericphase images. Unsuspected cirrhosis leading to rectal or small bowel varices is frequently encountered as the cause of OGIB in this initial assessment. Thereafter, a systematic interrogation of the small bowel is performed on each imaging phase beginning with the arterial phase and ending with the delayed phase. Visual inspection is performed to identify high attenuation structures, which can signify debris, neoplasm, vascular lesions, or active bleeding. Active bleeding is characterized by the progressive accumulation of intraluminal contrast, so identifying high-attenuation structures should immediately prompt comparison between phases to look for accumulation of intraluminal contrast, in addition to 2D and 3D morphologic assessment. Sometimes, jets of extravagated contrast will be identified at the site of active bleeding. Intraluminal debris will be unchanged between phases and often possesses sharp edges, and some movement of debris may occur between phases due to peristalsis. Similarly, intraluminal filling defects and focal bowel wall thickening are compared between phases and generally represent polyps, neoplasia, or Crohn’s disease; they are described subsequently. Three-dimensional interpretation using maximum intensity projection (MIP) images and interactive volume renderings (VR) is particularly helpful in the arterial phase, which will assist with identifying dilated feeding arteries and early draining veins.