David P. Wood, Jr., MD
Benign Tumors of the Bladder
Epithelial Metaplasia
Epithelial metaplasia is focal areas of transformed urothelium with normal nuclear and cellular architecture surrounded by normal urothelium usually located on the trigone and composed of squamous (squamous metaplasia) or glandular (glandular metaplasia) cells. Squamous metaplasia often has a knobby appearance and is covered by white, flaky, easily disrupted material lying on the trigone. Glandular metaplasia appears as clumps of raised red areas that appear inflammatory and are often confused for cancer. Approximately 40% of women and 5% of men have squamous metaplasia of the bladder, which is usually related to infection, trauma, and surgery (Ozbey et al, 1999). There are no racial differences, and squamous metaplasia is more common in women of childbearing age. Spinal cord injury is associated with squamous metaplasia most likely from catheter trauma and urinary tract infections (Vaidyanathan et al, 2003). Glandular metaplasia can extensively involve the bladder, particularly the trigone, but biopsy is not required. Treatment is unnecessary, and a preventive agent has not been identified.
Leukoplakia
Leukoplakia of the bladder is similar to squamous metaplasia with the addition of keratin deposition that appears as a white flaky substance floating in the bladder (Staack et al, 2006). Leukoplakia occurs in other organs that are covered by squamous epithelium and is often premalignant (Zhang et al, 2009). However, cytogenetic studies on bladder leukoplakia are consistent with a benign lesion, and no treatment is necessary (Staack et al, 2006).
Inverted Papilloma
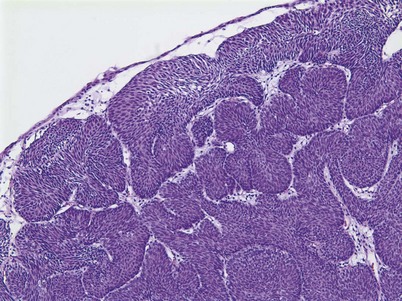
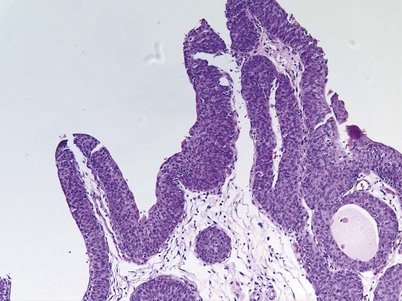
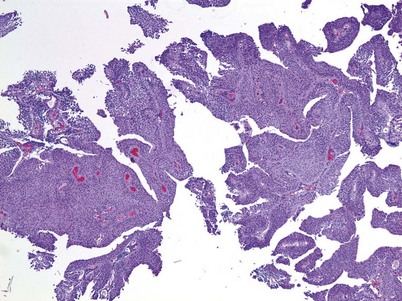
An inverted papilloma is a benign proliferative lesion that is associated with chronic inflammation or bladder outlet obstruction and can be located throughout the bladder but most commonly on the trigone, comprising less than 1% of all bladder tumors (Sung et al, 2006; Jones et al, 2007; Kilciler et al, 2008). Inverted papillomas demonstrate an inverted growth pattern composed of anastomosing islands of histologically and cytologically normal urothelial cells invaginating from the surface urothelium into the lamina propria but not into the muscularis propria (Fig. 80–1) (Sung et al, 2006). When diagnosed according to strictly defined criteria (e.g., lack of cytologic atypia), inverted papillomas behave in a benign fashion with only a 1% incidence of tumor recurrence (Sung et al, 2006; Kilciler et al, 2008). Occasionally, inverted papillomas are present with coexistent urothelial cancer elsewhere in the urinary system, occurring more commonly in the upper tract than the bladder (Asano et al, 2003). The use of fluorescent in-situ hybridization (FISH) to evaluate chromosomal changes can distinguish between an inverted papilloma and a urothelial cancer with an inverted growth pattern (Jones et al, 2007). Transurethral resection is the treatment of choice.
Papilloma
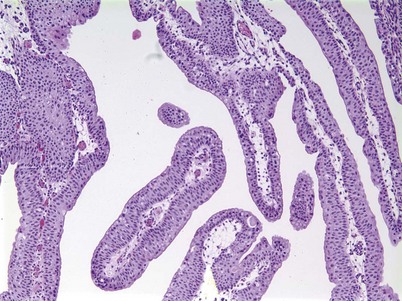
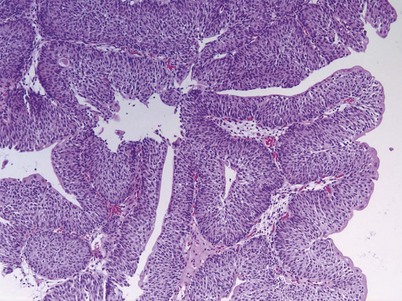
Urothelial papilloma is a benign proliferative growth in the bladder that is composed of delicate stalks lined by normal-appearing urothelium (Fig. 80–2) (Montironi and Lopez-Beltran, 2005). Papillomas had previously been categorized as grade 1 Ta tumors of the bladder until the World Health Organization (WHO) changed the classification of noninvasive bladder cancer in 1998 (Epstein et al, 1998). Papillomas rarely have mitotic figures and lack markers of aggressive growth such as TP53 or RB mutations, but 75% of these tumors will have mutations in the fibroblast growth factor receptor-3 (FGFR-3) (van Rhijn et al, 2004). Papillomas may recur, but they do not progress or invade.
Nephrogenic Adenoma
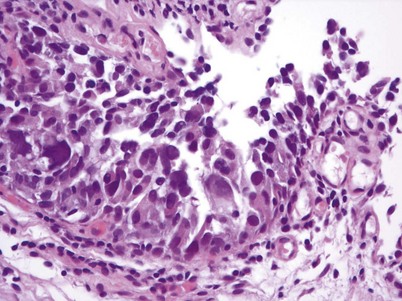
Nephrogenic adenoma is a rare tumor caused by chronic irritation of the urothelium; it arises from a variety of sources, including trauma, previous surgery, renal transplantation, intravesical chemotherapy, stones, catheters, and infection (Wood et al, 1988; Tse et al, 1997). Nephrogenic adenoma is composed of glandular-appearing tubules similar to renal tubules that involve the mucosa and submucosa of the bladder. These structures are covered by cuboidal cells with clear or eosinophilic cytoplasm with cytologically normal nuclei. The lesion may be vascular, which explains the presence of gross hematuria in most cases (Porcaro et al, 2001). There is no racial or gender association with the entity. The constant theme of chronic inflammation suggests metaplastic changes to the urothelium lead to nephrogenic adenoma, though some authors have proposed a theory that nephrogenic adenoma arises from nests of displaced mesonephric tissue in the urothelium that is activated with mucosal injury (Porcaro et al, 2001). The most frequent presenting symptom is gross hematuria, often in conjunction with a urinary tract infection. Treatment consists of transurethral resection and elimination of the chronic irritation.
Cystitis Cystica and Glandularis
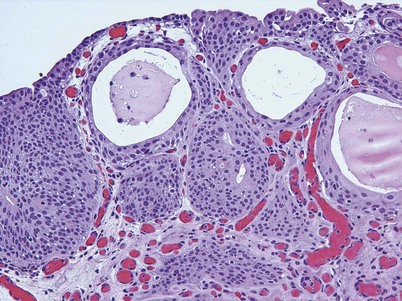
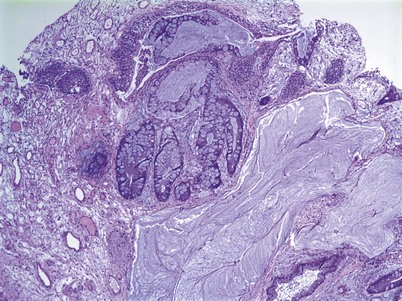
Cystitis cystica and/or glandularis is a common finding in normal bladders, usually associated with inflammation or chronic obstruction (Semins and Schoenberg, 2007). These benign tumors represent cystic nests that are lined by columnar or cuboidal cells and are generally associated with proliferation of Von Brunn nests (Figs. 80-3 and 80-4). Cystitis glandularis can be associated with pelvic lipomatosis and may occupy the majority of the bladder (Buckley et al, 2007). Cystitis glandularis may develop into or coexist with intestinal metaplasia, which are benign tumors characterized by goblet cells that are histologically similar to colonic epithelium. There have been a few case reports of cystitis cystica or glandularis transforming into adenocarcinoma, and therefore regular endoscopic evaluation of patients with these entities is recommended (Smith et al, 2008). The most common presenting feature of cystitis cystica or glandularis is irritative voiding symptoms and hematuria. Treatment is transurethral resection and relief of the obstruction or inflammatory condition.
Leiomyoma
Leiomyomas are the most common nonepithelial benign tumor of the bladder composed of benign smooth muscle. Several hundred cases have been reported, but this may under-represent the true prevalence. These tumors occur most commonly in women of childbearing age and are histologically similar to leiomyomas of the uterus (Castillo et al, 2008). Leiomyomas appear as smooth indentations of the bladder and can be confused with a bladder tumor except for the normal urothelium overlying the tumor (Fasih et al, 2008). Imaging, especially with magnetic resonance imaging (MRI), can confirm the diagnosis and spare invasive procedures (Fasih et al, 2008). Surgical resection is required if the leiomyoma is large or painful.
Urothelial Cancer
Epidemiology
The incidence rate of a cancer is defined as the number of new cancers diagnosed per 100,000 persons per year. The prevalence rate is the total number of cancers per 100,000 persons per year, not just new cases. Because urothelial cancer is a cancer of the environment and age, the incidence and prevalence rates increase with age, peaking in the 8th decade of life, and there is a strong association between environmental toxins and urothelial cancer formation (Jemal et al, 2008; Parkin, 2008). The incidence rate of urothelial cancer has been rising over the last 60 to 70 years, but the rate of rise has recently decreased significantly and in some geographic areas has leveled off (Parkin, 2008). Unfortunately, the incidence rate is rising the fastest in underdeveloped countries where industrialization has led to carcinogenic exposure.
According to the latest American Cancer Society statistics, there were 68,810 total cases diagnosed in the United States in 2007, comprising 51,230 men and 17,580 women and accounting for 7% of all cancers (Jemal et al, 2008). Bladder cancer is a lethal disease with 14,100 deaths recorded in 2007, comprising 9950 men and 4150 women and accounting for 3% of all cancer deaths. Importantly, there has been a 5% decrease in bladder cancer mortality from 1990 to 2004 despite a continued rise in the incidence of the disease. Bladder cancer contributes to 0.7% of the absolute decrease in the cancer death rate seen during this time, when all cancers are considered (Jemal et al, 2008). Globally, the incidence rate of bladder cancer has been increasing, but due to smoking cessation programs, at a slower rate over the last decade (Parkin, 2008). The mortality rate from bladder cancer has decreased significantly from 1990 to 2004 with both men and women recording a 18.4% decrease in mortality rate (Fig. 80–5). This decrease in mortality rate is more striking in men than women because of the earlier peak when men begin to smoke, which occurred approximately 20 years before women. Because of the latency period with urothelial cancer–causing agents in tobacco, we should see a commensurate decrease in the mortality rate in women in 15 to 20 years as their smoking cessation programs become more widespread.
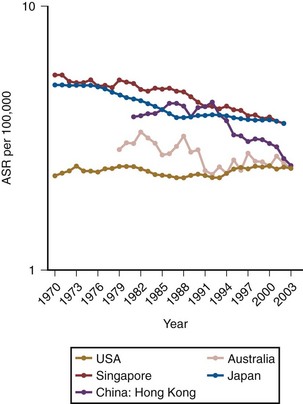
Figure 80–5 Age-Standardized Rates (ASR) of global mortality from bladder cancer.
(From Parkin DM. The global burden of urinary bladder cancer. Scand J Urol Nephrol Suppl 2008;218:12–20.)
Gender/Racial/Age Differences
Males are 3 to 4 times more likely to develop bladder cancer than females, presumably because of an increased prevalence of smoking and exposure to environmental toxins (Jemal et al, 2008; Parkin, 2008). African-American males have a 19% higher incidence rate than white males for all cancers and a 37% higher death rate. However, for urothelial cancer, white males have a higher incidence and death rate than African-Americans (Jemal et al, 2008; Parkin, 2008). African-American women have a 6% lower incidence but a 17% higher death rate than white women in all cancers (Jemal et al, 2008). However, bladder cancer is roughly 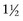 times more common in white women than in African-American women. A white male has a 3.7% chance of developing urothelial cancer in his lifetime, which is roughly 3 times the probability for white females or African-American males and more than
times more common in white women than in African-American women. A white male has a 3.7% chance of developing urothelial cancer in his lifetime, which is roughly 3 times the probability for white females or African-American males and more than 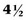 times the probability for African-American females (Hayat et al, 2007; Jemal et al, 2008). The risk of developing invasive bladder cancer is age dependant (Jemal et al, 2008). For men from birth to age 39 years, the incidence rate of invasive bladder cancer is 0.02%; ages 40 to 59 years, 0.41%; ages 60 to 69 years, 0.96%; ages 70 years and older, 3.5%; and from birth to death, 3.7%. The bladder cancer incidence for women from birth to age 39 years is 0.1%; ages 40 to 59 years, 0.13%; ages 60 to 69 years, 0.26%; ages 70 years and older, 0.99%; and from birth to death, 1.17%. In general, adolescents and young adults (less than age 40 years) tend to develop well-differentiated noninvasive, rather than invasive, bladder cancer (Linn et al, 1998). Unlike many other cancers where younger patients tend to develop more aggressive disease, the opposite appears to be true in bladder cancer, because they present more frequently with noninvasive low-grade tumors. However, the death rate, stage for stage, from bladder cancer is the same across all age groups (Wan and Grossman, 1989).
times the probability for African-American females (Hayat et al, 2007; Jemal et al, 2008). The risk of developing invasive bladder cancer is age dependant (Jemal et al, 2008). For men from birth to age 39 years, the incidence rate of invasive bladder cancer is 0.02%; ages 40 to 59 years, 0.41%; ages 60 to 69 years, 0.96%; ages 70 years and older, 3.5%; and from birth to death, 3.7%. The bladder cancer incidence for women from birth to age 39 years is 0.1%; ages 40 to 59 years, 0.13%; ages 60 to 69 years, 0.26%; ages 70 years and older, 0.99%; and from birth to death, 1.17%. In general, adolescents and young adults (less than age 40 years) tend to develop well-differentiated noninvasive, rather than invasive, bladder cancer (Linn et al, 1998). Unlike many other cancers where younger patients tend to develop more aggressive disease, the opposite appears to be true in bladder cancer, because they present more frequently with noninvasive low-grade tumors. However, the death rate, stage for stage, from bladder cancer is the same across all age groups (Wan and Grossman, 1989).
Global Burden of Bladder Cancer
There is a geographic difference in bladder cancer incidence rates across the world with the highest occurring in Southern and Eastern Europe parts of Africa, the Middle East, and North America and the lowest occurring in Asia and underdeveloped areas in Africa (Ferlay et al, 2007). Bladder cancer is the 9th most common cancer worldwide, with 357,000 cases recorded in 2002 (Parkin, 2008). Bladder cancer is the 13th most common cause of death, accounting for 145,000 deaths worldwide (Ferlay et al, 2007; Parkin, 2008). The incidence rate of bladder cancer has been rising in Asia and Russia because of an increased prevalence of smoking. Sixty-three percent of all bladder cancer cases occur in developed countries, with 55% from North America and Europe. In the United States, the highest bladder cancer incidence rate is in Rhode Island and the lowest is in the District of Columbia (Jemal et al, 2008). The histologic cell type of bladder cancer is very geographically dependent, but urothelial cancer is the most common. In North America and Europe, 95% to 97% of cases are urothelial carcinoma; in Africa 60% to 90% are urothelial and 10% to 40% are squamous cell; and Egypt has the highest rate of squamous cell carcinoma because of the endemic infections with Schistosoma species (Parkin, 2008).
Mortality
The mortality rate from bladder cancer in Egypt is 3 times higher than in Europe and 8 times greater than in North America because of the aggressive nature of squamous cell carcinoma that is highly prevalent in Egypt (Parekh et al, 2002). In the United States, death rates for all cancer sites combined decreased by 2.6% per year in males and by 1.8% in females from 2002 to 2004 compared with 1.5% and 0.8% per year in males and females, respectively, from 1992 to 2002 (Jemal et al, 2008). The mortality rate for bladder cancer has decreased by 5% during this period primarily because of smoking cessation, changes in environmental carcinogens, and healthier lifestyles. Mortality from bladder cancer is highest in elderly persons, particularly those over the age of 80 years, accounting for the third most common cause of cancer deaths in men older than age 80 years (Jemal et al, 2008). Whether this increase in mortality rate is related to tumor biology or changes in physician practices with the elderly is unclear. Recent evidence suggests that physician practices may be related to bladder cancer deaths in the elderly (Morris et al, 2009). These authors estimated that 31% of all bladder cancer deaths were avoidable, more commonly in noninvasive rather than invasive disease. Better chemotherapy has improved the survival rate in patients with metastatic bladder cancer, but changes in physician practices related to more timely care and more aggressive treatment in healthy patients could well lead to improvement in overall survival. Lee and colleagues (2006) reported that a delay of more than 12 weeks from the diagnosis of bladder cancer to cystectomy treatment was associated with a decrease in overall and cancer-specific survival. However, more intensive treatment (intravesical chemotherapy and cystoscopy) for patients with noninvasive bladder cancer did not correlate with the better survival or less need for invasive treatment (Hollenbeck et al, 2009; Morris et al, 2009).
Key Points: Epidemiology
Genetic
The higher incidence of bladder cancer in white compared with African-American males is probably not genetic but environmental, or it may be related to differential susceptibility to carcinogens (Bouchardy et al, 1995, 1996; Wanner et al, 1995). There are several polymorphisms that seem to be related to the formation of bladder cancer, in particular the susceptibility to environmental carcinogens. N-acetyl transferase (NAT) detoxifies nitrosamines, a known bladder carcinogen. Specifically, NAT-2 regulates the rate of acetylation of compounds such as caffeine, which are related to bladder cancer formation. The slow NAT-2 polymorphism is related to bladder cancer with an odds ratio of 1.4 compared with the fast polymorphism (Garcia-Closas et al, 2005). Glutathione-S-transferase (GSTM1) conjugates several reactive chemicals, including arylamines and nitrosamines. The null GSTM1 polymorphism is associated with an increased bladder risk with a relative risk of 1.5 (Garcia-Closas et al, 2005). The null GSTM1 and slow NAT-2 lead to high levels of 3-aminobiphenyl and higher risk of bladder cancer. These polymorphisms are present in 27% of white, 15% of African-American, and 3% of Asian males, thus partially explaining the different bladder cancer incidence rates across ethnic groups.
External Risk Factors
In addition to the skin and lungs, the bladder is the main internal organ affected by occupational carcinogens. The primary culprits are the aromatic amines that bind to DNA (Delclos and Lerner, 2008; Reulen et al, 2008). Twenty percent to 27% of all bladder cancers are associated with industrial exposure of some type, primarily in areas with a heavy concentration of chemical industries (Case and Hosker, 1954; Blot and Fraumeni, 1978; Reulen et al, 2008). Among the first chemical agents implicated in the formation of bladder cancer in dye and rubber workers were benzidine and β-naphthylamine (Case and Hosker, 1954). Activation of these amines to allow DNA binding occurs with enzymes that are selectively expressed in the population, making some subjects more susceptible to cancer formation as described above for the NAT-2 and GSTM1 polymorphisms. There are 11 specific aromatic amines implicated in bladder cancer formation; they are broken into three groups—definite, probable, and possible—as seen in Table 80–1. Other industrial agents implicated in bladder cancer formation include polycyclic aromatic hydrocarbons (PAH), diesel exhaust, and paint substances (Zeegers et al, 2001a).
Table 80–1 Aromatic Amines Associated with Urothelial Cancer Formation
| DEFINITE | PROBABLE | POSSIBLE |
|---|---|---|
| 4-Chloro-ortho-toluidine |
Data from Delclos GL, Lerner SP. Occupational risk factors. Scand J Urol Nephrol Suppl 2008;218:58–63; and IARC. Tobacco smoke and involuntary smoking. Lyon (France): IARC Press; 2004.
Environmental carcinogens can enter the system and cause bladder cancer from inhalation or through skin absorption. In general, there is a long latency period of 10 to 20 years between the industrial exposure and the formation of the bladder cancer, thus proving definitive causative relationships is difficult (Dryson et al, 2008). However, there are a variety of occupations statistically associated with bladder cancer formation, and all are industrial in nature (Table 80–2) (Reulen et al, 2008). The overall increased risk of bladder cancer formation in industrial workers is 30%, with agriculture workers having the lowest and rubber workers the highest risk of bladder cancer formation.
Table 80–2 Relative Risk of Occupations Associated with Urothelial Cancer Formation
| OCCUPATION | RR (95% CI) |
|---|---|
| Armed forces | 1.09 (0.94-1.26) |
| Managers | 1.17 (0.96-1.44) |
| Architects and engineers | 1.07 (0.91-1.25) |
| Health professionals | 1.02 (0.92-1.13) |
| Nurses | 1.07 (0.91-1.26) |
| Teaching professionals | 0.90 (0.74-0.09) |
| Writers and artists | 1.20 (1.05-1.36) |
| Clerks | 1.01 (0.92-1.10) |
| Cooks | 1.14 (0.86-1.50) |
| Hairdressers | 1.23 (1.11-1.37) |
| Protective service occupations | 1.07 (0.96-1.19) |
| Firefighters | 1.17 (0.92-1.49) |
| Police officers and guards | 1.10 (0.95-1.29) |
| Agricultural workers | 0.86 (0.79-0.93) |
| Gardeners | 1.04 (0.78-1.40) |
| Forestry workers | 0.93 (0.73-1.17) |
| Fishery workers | 0.95 (0.83-1.07) |
| Miners | 1.31 (1.09-1.57) |
| Building-frame workers | 1.06 (0.97-1.16) |
| Bricklayers | 1.03 (0.97-1.10) |
| Carpenters | 1.04 (0.90-1.21) |
| Building finishers | 1.10 (1.05-1.15) |
| Plumbers | 1.14 (1.03-1.25) |
| Electricians | 1.07 (1.02-1.13) |
| Painters | 1.17 (1.10-1.23) |
| Metalworkers | 1.10 (1.02-1.20) |
| Welders | 1.09 (0.98-1.20) |
| Sheet-metal workers | 1.14 (0.98-1.33) |
| Blacksmiths and toolmakers | 1.16 (1.06-1.26) |
| Blacksmiths | 1.27 (1.02-1.58) |
| Toolmakers | 1.10 (0.90-1.34) |
| Machine-tool setters | 1.24 (1.09-1.42) |
| Mechanics | 1.21 (1.12-1.31) |
| Motor mechanics | 1.27 (1.10-1.46) |
| Glassmakers | 1.31 (0.94-1.81) |
| Printers | 1.13 (0.96-1.33) |
| Food processors | 1.02 (0.93-1.11) |
| Butchers | 0.99 (0.78-1.26) |
| Bakers | 1.05 (0.87-1.26) |
| Cabinetmakers | 1.10 (0.94-1.27) |
| Textile workers | 1.12 (0.97-1.29) |
| Tailors | 1.28 (0.99-1.65) |
| Leather workers | 1.27 (1.07-1.49) |
| Machinist | 1.18 (1.06-1.30) |
| Metal processors | 1.18 (1.06-1.32) |
| Furnace operators | 1.17 (0.96-1.42) |
| Paper-pulp workers | 1.08 (0.90-1.29) |
| Petroleum workers | 1.15 (0.97-1.36) |
| Rubber workers | 1.29 (1.06-1.58) |
| Motor vehicle drivers | 1.11 (1.06-1.17) |
| Car, taxi, and van drivers | 1.20 (1.03-1.39) |
| Bus drivers | 1.29 (1.08-1.53) |
| Truck drivers | 1.18 (1.06-1.33) |
| Domestic helpers | 1.19 (1.01-1.42) |
| Cleaners | 1.06 (0.80-1.41) |
| Launderers | 1.27 (0.95-1.71) |
| Building caretakers | 1.17 (0.92-1.49) |
| Hand packers | 1.07 (0.93-1.23) |
| Freight handlers | 1.03 (0.96-1.11) |
| Dye makers | 1.10 (1.06-1.13) |
| Sales workers | 1.05 (0.98-1.13) |
| Woodworkers | 1.05 (0.89-1.24) |
| Chemical workers | 1.19 (0.98-1.43) |
CI, confidence interval; RR, relative risk.
From Reulen RC, Kellen E, Buntinx F, et al. A meta-analysis on the association between bladder cancer and occupation. Scand J Urol Nephrol Suppl 2008;218:64–78.
Smoking
Tobacco is the main known cause for urothelial cancer formation, particularly cigarette smoking, accounts for 60% and 30% of all urothelial cancers in males and females, respectively (Brennan et al, 2000, 2001; Boffetta, 2008; Gandini et al, 2008). The relative risk of developing urothelial cancer from smoking is 2.8 and 2.73 in men and women, respectively (Gandini et al, 2008). Overall there is a 2- to 6-times greater chance of developing urothelial cancer with smoking, and the intensity and duration of smoking is linearly related to the increased risk, with no clear plateau level (Brennan et al, 2000; Smoke, 2004; Boffetta, 2008). If a person smokes 1 to 9 cigarettes versus more than 21 cigarettes per day, the relative risk of bladder cancer is 1.5 versus 5.4, respectively (Weir and Dunn, 1970). If a person smokes 1 to 10 years versus more than 40 years, the relative risk of bladder cancer is 1.2 versus 3.0, respectively (Burns and Swanson, 1991). If a person smokes more than 60 years, they have a sixfold increased risk of developing urothelial cancer compared with a nonsmoker (Burch et al, 1989). The type of tobacco smoked appears to be associated with bladder cancer formation because of different carcinogens present within the tobacco. Black tobacco appears to be worse than blonde tobacco because of a greater amount of aromatic amines in the former (Boffetta, 2008). Cigars and pipes are probably associated with bladder cancer formation, but there are too few studies evaluating only cigar and pipe smokers because of the high probability that these subjects also smoke cigarettes. The risk of bladder cancer formation in those who chew tobacco is unclear. Again, most studies did not restrict their subjects to just chewing tobacco, but in general, there is an increased risk of bladder cancer formation associated with chewing tobacco. The risk of secondhand smoke in bladder cancer formation is low and not statistically different than that for nonsmokers (Zeegers et al, 2002). Importantly, smoking cessation does make a difference in urothelial cancer formation. Smokers who have stopped for 1 to 3 years have a 2.6 relative risk, and those stopping for more than 15 years have a 1.1 relative risk of bladder cancer formation (Wynder and Goldsmith, 1977; Smoke, 2004). Smoking is responsible for 30% of all deaths from bladder cancer in males and accounts for 46% of all bladder cancer deaths in high-income countries and 28% in low- to middle-income countries (Brennan et al, 2000; Parkin, 2008). The impact of smoking on mortality is more startling in women, in whom smoking is responsible for 13% of bladder cancer deaths, with 29% in high-income countries compared with only 3% in low- to middle-income countries.
Nutritional Factors
Most nutrients or other metabolites are excreted in the urine and have prolonged contact with the urothelium, particularly in the bladder; therefore nutrition plays a role in urothelial cancer formation (Steinmaus et al, 2000; Brinkman and Zeegers, 2008). However, there are inconsistent reports regarding the exact fruits and vegetables that are beneficial in preventing urothelial cancer, suggesting that epidemiologic factors are at play. In general, a Mediterranean diet leads to the lowest urothelial cancer risk. In a case-controlled study, there were fewer cases of urothelial cancer in the group given a Mediterranean diet versus a standard Western diet, probably because of the increased ingestion of fruits and vegetables (de Lorgeril et al, 1998). Both fruits and vegetables—specifically citrus, apples, berries, tomatoes, carrots, and cruciferous vegetables—contain several active compounds that are important in detoxification. These compounds include polyphenols, antioxidants, and enzymes that modulate the detoxification of nitrous amines and can prevent DNA adduct formation and oxidative damage (Boffetta, 2008; De Stefani et al, 2008; Lumbreras et al, 2008). Micronutrients associated with a preventive effect on urothelial cancer formation are mainly antioxidants, including vitamins A, C, and E; selenium; and zinc (Michaud et al, 2000; Zeegers et al, 2002; Schabath et al, 2005; Brinkman and Zeegers, 2008). Fish, rice, and cereal do not seem to have a protective or detrimental effect on urothelial cancer formation (Radosavljevic et al, 2005). Foods that have an indeterminate effect on bladder cancer formation include beef, eggs, and processed meats (Balbi et al, 2001; Wakai et al, 2004; Brinkman and Zeegers, 2008). Nutritional factors that have been associated with causing or promoting the formation of urothelial cancer include salted and barbequed meat, pork, total fat, pickled vegetables, soy, and spices (Balbi et al, 2001).
Occurrence of urothelial cancer is moderately higher in coffee and tea drinkers, but this may be compounded by smoking or other dietary factors associated with people who drink coffee or tea (Pelucchi et al, 2008). There is no apparent intensity or duration association of coffee and tea ingestion, suggesting an indirect causative effect, unlike for smoking. Coffee and tea do not appear to have any carcinogenic effect in animals; however, one study did show a twofold increase in urothelial cancer formation in nonsmokers who drank large amounts of coffee in western New York (Vena et al, 1993a). Conversely, a pooled analysis of 10 studies of nonsmokers related to coffee or tea ingestion showed no increased risk of cancer formation in coffee or tea drinkers (Sala et al, 2000). In conclusion, there are inconsistencies regarding nutritional factors related to urothelial cancer formation, in part because of confounding effects and associations, including coffee ingestion and smoking, ingestion of fruits and vegetables without involvement of smoking, and epidemiologic factors. However, even if not directly causative, there is a very clear association between a healthy diet and a decreased risk of urothelial cancer formation.
Fluid Intake
Urogenesis theory prescribes that decreased fluid intake leads to less micturition and higher concentrations of potential carcinogens in the urine and thus an increased risk of bladder cancer (Braver et al, 1987). A meta-analysis concluded that approximately 50% of the studies on fluid intake and bladder cancer risk showed an association and 50% did not find an association (Brinkman and Zeegers, 2008). Adding to the controversy, Vena and colleagues (1993b) reported a decreased risk of bladder cancer with increased fluid intake in women, but the opposite was true in men. In conclusion, although it makes sense that increased fluid intake would decrease the concentration of potential carcinogens and thus decrease the risk of bladder cancer, the scientific data supporting this theory is inconclusive.
Alcohol
Alcohol intake is related to several cancers, including those of the oral cavity, esophagus, liver, larynx, and breast, accounting for 3.6% of all cancers (Boffetta and Hashibe, 2006; Pelucchi et al, 2008). However, a meta-analysis of available literature from the last 20 years did not show an association between alcohol intake and bladder cancer, with a relative risk of 1.2 overall—1.3 in men and 1.0 in women (Zeegers et al, 2001b).
Artificial Sweeteners
Some animal studies have shown that large doses of saccharine or cyclamates may influence the development of bladder cancer (Allen et al, 1957; Sontag, 1980). These studies are controversial because of the high doses of saccharine and cyclamates provided to the animals and the altered composition of these compounds, which may have influenced the carcinogenic activity found in animal studies (Cohen et al, 1995). Epidemiologic studies in humans have shown no evidence of an increased risk of bladder cancer in consumers of artificial sweeteners (Armstrong and Doll, 1975; Morrison et al, 1982).
Analgesic Abuse
Acetaminophen is the active metabolite of phenacetin, a commonly used antipyretic and analgesic. Consumptions of large quantities of acetaminophen or phenacetin (5 to 15 kg during a 10-year period) have been associated with an increased risk of renal cancer and, perhaps, bladder cancer (Piper et al, 1985). However, these studies relied on interviews and questionnaires to ascertain drug exposure rather than actual determination of analgesic use. Kaye and colleagues (2001) performed a nested match case–controlled study and found no association between acetaminophen or other nonsteroidal anti-inflammatory drug ingestion and bladder cancer.
Inflammation/Infection
Infection is clearly a contributor to the formation of squamous cell carcinoma in patients chronically infected with Schistosoma hematobium and will be covered in the section on squamous cell carcinoma of the bladder (Abol-Enein, 2008). There is a possible link between human papillomavirus (HPV) and urothelial cancer formation. HPV encodes two oncoproteins, E6 and E7. E6 interacts with TP53, which has a role in bladder cancer progression and formation (Westenend et al, 2001). A meta-analysis supports a possible association between HPV infection and bladder cancer, reporting a 2.3 relative risk with confidence intervals of 1.3 to 4.1 (Gutierrez et al, 2006). However, the association between HPV and bladder cancer depends significantly on the method of analysis, the statistical evaluation of the data, proving infection status, and recall bias by the individual. The BK virus is oncogenic in newborn hamsters and can immortalize mammalian cells in vitro (Newton et al, 2005). BK virus can cause severe hemorrhagic cystitis in bone marrow transplant recipients; however, there is no consistent link between BK virus infection and urothelial carcinoma.
Bacterial
Several investigators have suggested that chronic bacterial infections may play a role in bladder cancer formation (Davis et al, 1991). Clinically, chronic catheter use, stones, and infections are associated with bladder carcinoma, but the mechanism of neoplastic formation is not well understood (Abol-Enein, 2008). The mechanism of action may be related to the production of carcinogens like nitrosamines that can be produced with chronic urinary tract infections (Radomski et al, 1978). In an animal model, rats with a urinary tract infection produce increased urinary levels of N1 N-dimethylnitrosamine over a 24-week period, and this was associated with urothelial hyperplasia and early neoplastic changes in the urothelium. The potential carcinogens were produced primarily with infections caused by Escherichia coli and Pseudomonas. A retrospective review of published literature suggests that chronic urinary tract infections are associated with bladder cancer, reporting a 14 to 16 relative risk of developing bladder cancer for any history of urinary tract infection versus none (Abol-Enein, 2008). However, there has been no prospective study examining the association between urinary tract infections and bladder cancer risk. It remains possible that the positive association between infection and urothelial cancer is driven by detection bias or preferential recall between cases and controls. Finally, a large case control study from the United States based on data from the National Bladder Cancer Study Group reported a 4.8 relative risk (CI, 1.9 to 11.5) of bladder cancer formation for subjects with greater than or equal to three urinary tract infections versus none (Kantor et al, 1984).
Previous gonorrhea infections have been associated with urothelial cancer formation. In a case-controlled study adjusting for age, gender, smoking, and other confounders, the relative risk of urothelial cancer formation was 2.1 to 2.4 (La Vecchia et al, 1991). A prospective study found a 1.92 relative risk (CI, 1.0 to 3.3) of bladder cancer formation in men with a history of gonorrhea (Michaud et al, 2007). Previous gonorrhea infection was most associated with invasive rather than noninvasive cancers. These studies suggest that gonorrhea infection may be associated with bladder cancer formation, and further prospective studies would be warranted.
Radiation
The potential association between radiation exposure and bladder cancer formation is primarily based on atomic bomb survivors during World War II (Ron et al, 1994; Thompson et al, 1994; Pierce et al, 1996; Hall, 2008). Since 1950, 86,572 people who were exposed to atomic bomb radiation have been followed. Seventy-three percent had a low dose of exposure (less than 50 mSv), and 6% had exposure to very high doses of radiation (more than 500 mSv). There is a significant increased risk of dying from any cancer if a person is exposed to greater than 50 mSv. For urothelial cancer, the relative risk of urothelial cancer formation is 1.63 in men and 1.74 in women. Interestingly, urothelial cancer formation after radiation is not age related, but the latency period is 15 to 30 years. Further support that radiation can cause bladder cancer is an increased risk of urothelial cancer in those patients with prostate or cervical cancer who were treated with radiation therapy (Boice et al, 1988; Neugut et al, 1997; Brenner et al, 2000). Even the radiation from more than six CT scans in a given year is associated with second malignancies (Hansen and Jurik, 2009). However, there is no association with low-dose or industrial exposure of radiation therapy and bladder cancer formation. Importantly, urologic technicians or nuclear radiation workers do not have an increased risk of urothelial cancer formation (Sont et al, 2001; Mohan et al, 2003; Sigurdson et al, 2003).
Chemotherapy
Chemotherapy destroys malignant cells by causing significant DNA and cellular damage, but can also have a profound effect on rapidly dividing normal epithelium such as in the bladder. The only chemotherapeutic agent that has been proven to cause bladder cancer is cyclophosphamide (Travis et al, 1995; Nilsson and Ullen, 2008). The risk of bladder cancer formation is linearly related to the duration and intensity of cyclophosphamide treatment, supporting a causative role. Phosphoramide mustard is the primary mutagenic metabolite that causes bladder cancer in patients exposed to cyclophosphamide.
Heredity
First-degree relatives of patients with bladder cancer have a twofold increased risk of developing urothelial cancer themselves, but high-risk of urothelial cancer families are relatively rare (Aben et al, 2002; Murta-Nascimento et al, 2007; Kiemeney, 2008). The hereditary risk seems to be higher for women and nonsmokers, but it is not related to secondhand exposure to smoking in families. The inherited risk of bladder cancer formation appears to affect all stages of urothelial carcinoma and is not associated with bladder cancer formation at an earlier age. Unfortunately, there are no clear mendelian inheritance patterns, making classic linkage studies impossible. Most likely, there are a variety of low-penetrance genes that can be inherited to make a person more susceptible to carcinogenic exposure, thus increasing the risk of bladder cancer formation. There are two high-penetrance genes that are associated with urothelial carcinoma: RB1 and CDC91L1 (Fletcher et al, 2004; Guo et al, 2004). In a recent study, there were five bladder cancer cases from 144 patients with known retinoblastoma, resulting in a relative risk of 26.3 compared with those without retinoblastoma (Fletcher et al, 2004). CDC91L1 was discovered in 1996 and is a germline translocation of t(5;20) (p15;q11) that is located on 20q11 (Guo et al, 2004). This gene encodes phosphatidylinositol glycan class U and is amplified in one third of urothelial carcinomas. The role of the gene is unclear, and the protein expressed is unknown at this time. Thelen and Schaeuble were the first to report familial clustering of urothelial carcinomas in 1957 by identifying identical twins with urothelial papillomas. This finding has led to further evaluations of looking for an inherited form of urothelial cancer, which to date has proven elusive.
Key Points: External Risk Factors
Pathology
Histologically, 90% of bladder cancers are of urothelial origin, 5% are squamous cell carcinomas, and less than 2% are adenocarcinoma or other variants (Lopez-Beltran, 2008). Urothelial carcinoma is the most common malignancy of the urinary tract and is the second most common cause of death among genitourinary tumors. At initial presentation, 80% of urothelial tumors are non–muscle invasive. There are multiple growth patterns of urothelial cancer, including flat carcinoma in situ (CIS), papillary tumors that can be low or high grade, and sessile tumors with a solid growth pattern. Non–muscle-invasive cancers can be very large because of lack of genetic alterations required for invasion. Likewise, invasive tumors can be quite small if early genetic changes occur within the tumor cell, allowing for an invasive phenotype.
The World Health Organization (WHO) first determined nomenclature for the histologic classification of urothelial cancer in 1973. In 1998, the International Society of Urological Pathology (ISUP) developed a new nomenclature to better reflect the recurrence and progression rates of urothelial cancer (Epstein et al, 1998). The two main changes were recognition that papillary Ta grade 1 urothelial cancers should not be considered cancers because of their indolent growth and lack of invasion, and the second was elimination of “grade 2” cancers, which became a grey zone encompassing grade 1 and grade 3 cancers causing interobserver variation. In 2004, the WHO adopted the ISUP recommended staging system and is the standard histologic nomenclature for urothelial carcinoma (Sauter et al, 2004). The clinical pattern of presentation is related to the cytologic and architectural alterations that occur within the tumor. Table 80–3 lists the histologic changes that occur from normal epithelium to high-grade muscle-invasive disease. Table 80–4 lists the neoplasms that can occur in the bladder, and Table 80–5 lists the WHO 2004 classification of urothelial neoplasms (Sauter et al, 2004; Montironi and Lopez-Beltran, 2005).
Table 80–3 Histologic Characteristics of Noninvasive Papillary Urothelial Tumors of the Bladder According to the WHO 2004 Classification
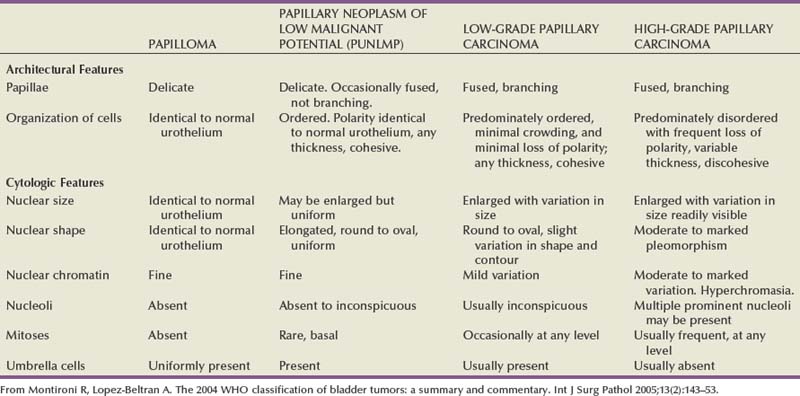
Table 80–4 Histologic Type of Tumors of the Urinary Bladder (World Health Organization, 2004)
NOS, not otherwise specified.
* Refers to tumors that are undifferentiated by light microscopy.
Lopez-Beltran A. Bladder cancer: clinical and pathological profile. Scand J Urol Nephrol Suppl 2008;218:95–109.
Table 80–5 2004 World Health Organization Classification of Noninvasive and Invasive Urothelial Neoplasia
| Noninvasive Urothelial Neoplasia |
| Invasive Urothelial Neoplasia |
From Montironi R, Lopez-Beltran A. The 2004 WHO classification of bladder tumors: a summary and commentary. Int J Surg Pathol 2005;13(2):143–53.
Precursor Lesions to Urothelial Cancer
Normal bladder urothelium is multilayered and less than seven cells thick (Epstein et al, 1998; Montironi and Lopez-Beltran, 2005) (Fig. 80–6). The cells mature from the basement membrane to the surface cells in an orderly fashion. The surface has large umbrella cells that may have nuclear atypia and form asymmetrical units. Their membrane is composed of uroplakin proteins and is rigid. These umbrella cells are part of the urine bladder barrier preventing toxins within the urine from transforming urothelial cells. Previously, normal urothelium was called “transitional cells” because of their ability to transition into other cell types, including squamous cells and adenocolumnar cells.
Precursor lesions are a continuum from hyperplasia to atypia to dysplasia and finally cancer. Different pathologists have their own interpretation of important cellular or structural changes that separate these histologic changes into different categories. Hyperplasia is characterized by markedly thicker mucosa with or without atypia (Fig. 80–7). The urothelium is more than seven cells thick, and there is some disorganization of the cellular architecture. Hyperplasia is often adjacent to low-grade tumors and thought to be a precursor lesion (Epstein et al, 1998). Loss of parts of chromosome 9 can occur in hyperplasia, particularly if adjacent to low-grade tumors (Hartmann et al, 1999). Urothelial hyperplasia appears as undulating or papillary growths that are best seen on flexible cystoscopy (Sauter et al, 2004). The presence of hyperplasia does not increase the risk of developing cancer; however, if present in someone with a history of urothelial carcinoma, it may herald a recurrence in the future (Montironi and Lopez-Beltran, 2005; Lopez-Beltran, 2008).
Reactive atypia can have nuclear changes associated with inflammation, regeneration, or reaction from noxious stimuli and is characterized by large cells with prominent nucleoli (Lopez-Beltran et al, 2002). Mitotic activity without abnormal forms can be present. Endoscopically, reactive atypia is edematous, especially in the lamina propria and associated with previous urinary instrumentation, stones, infection, or intravesical therapy (Lopez-Beltran, 2008).
Urothelial dysplasia has abnormal cytologic and nuclear changes that are preneoplastic but are not sufficient to be characterized as CIS (Sauter et al, 2004). Dysplasia is characterized by cohesive cells with mildly abnormal nuclear changes. There is nuclear crowding, prominent nucleoli, and abnormal mitotic figures may be present. Umbrella cells can be present with dysplasia, but not with CIS. Most cellular abnormalities are in the basal or early cell layers, with appropriate maturation toward the luminal surface. The urothelium is more than seven cells thick at times, but there can be sloughing of the more mature urothelium, exposing the less mature early cell layers. There is allelic loss of chromosome 9 and occasional TP53 abnormalities (Harnden et al, 1996; Hartmann et al, 1999). It is difficult to quantitate the clinical significance of dysplasia secondary to the interobserver reproducibility of this entity (Montironi and Lopez-Beltran, 2005). However, dysplasia is a good indication of urothelial instability and a marker of recurrence and progression in those with known urothelial cancer. Isolated dysplasia progressing to CIS occurs in approximately 19% of cases, but dysplasia in the face of previous history of urothelial cancer will form CIS in approximately 60% of cases (Cheng et al, 1999a).
Urothelial Cancer Histology
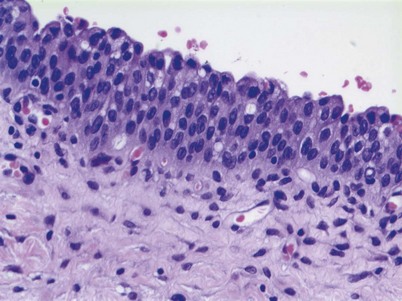
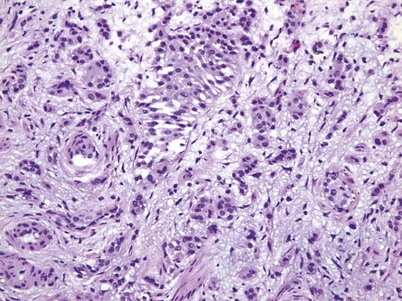
Non–muscle-invasive bladder cancer (NMIBC) includes CIS, papillary urothelial neoplasia of low-malignant potential (PUNLMP), and low- and high-grade urothelial cancer that previously had been called “superficial bladder cancer,” which is a misnomer. The clinical significance of the WHO grading classification is shown in Table 80–6. The grade distribution of NMIBC is 25% PUNLMP, 50% low grade, and 25% high grade (including CIS) (Holmang et al, 2001; Samaratunga et al, 2002). CIS is characterized as nonpapillary, flat, high-grade tumors in which the surface epithelium contains cancer cells (Fig. 80–8) (Sauter et al, 2004). There is severe nuclear atypia, loss of cellular polarity, and a noncohesive cellular structure. The cells are large, pleomorphic, chromatin clumping, and abnormal mitotic figures are common. Loss of umbrella cells is a characteristic, separating CIS from dysplasia. All CIS is high grade by definition. The genetic abnormalities associated with CIS include alterations to the RB, TP53, and PTEN genes (Cordon-Cardo et al, 2000; Lopez-Beltran et al, 2002; Cordon-Cardo, 2008). CIS is immunoreactive for cytokeratin 20, and NMP22 is present in the cells. CIS is a precursor lesion for invasive cancer and can spread to the distal ureters and prostatic urethra on the surface or in a pagetoid manner, undermining normal adjacent urothelium (Montironi et al, 2002). Endoscopically, CIS is reddish with heaped-up mucosa and can be mistaken for inflammatory changes or radiation cystitis. CIS in association with invasive tumors have a worse prognosis, with a 45% to 65% 5-year death rate (Lopez-Beltran et al, 2002).
Table 80–6 Clinical Significance of Different Non–Muscle-Invasive Urothelial Cancer Categories in WHO 2004 Grading System
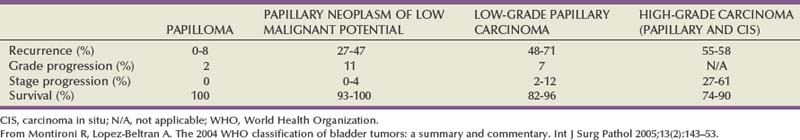
PUNLMP is a papillary growth with minimal cytological atypia that is more than seven cells thick and is generally solitary and located on the trigone (Fig. 80–9) (Holmang et al, 2001; Sauter et al, 2004). PUNLMP is composed of thin papillary stalks, where the polarity of the cells is maintained and the nuclei are minimally enlarged. PUNLMP has a low proliferation rate and is not associated with invasion or metastases, but almost 80% will have loss of chromosome 9 (Cheng et al, 2004). PUNLMP is different from a benign papilloma in that a PUNLMP has a thicker cell layer and large nuclei with occasionally mitotic figures. The male to female ratio for PUNLMP is 5 : 1, and the mean age is 65 (Holmang et al, 2001). PUNLMP can recur within the bladder in 35% of cases, but progression is rare, occurring in less than 4% (Oosterhuis et al, 2002).
Low-grade urothelial carcinoma is typically papillary in nature with a fibrovascular stalk and frequent papillary branching with increased cellular size, some nuclear atypia (more than in PUNLMP), and occasional mitotic figures (Fig. 80–10) (Epstein et al, 1998). Genetic abnormalities associated with low-grade cancer include deletion of 9q and alterations of FGFR-3, HRAS, and PI3K (Holmang et al, 2001; Cordon-Cardo, 2008). Low-grade carcinomas are immunoreactive for cytokeratin-20 and CD-44. The architectural and histologic changes that separate low-grade urothelial carcinoma from a PUNLMP include multiple stalks, more cytologic atypia, and the multifocal nature of low-grade carcinomas compared with the solitary PUNLMP.
High-grade papillary urothelial cancer is composed of fused papillary stalks with high-grade cancer in the urothelial layer. There is a disordered growth pattern, numerous mitotic figures present, and there are pleomorphic cells with exaggerated nuclei. Over 80% of high-grade cancers will invade the underlying stroma if left untreated. Genetic abnormalities associated with high-grade papillary urothelial cancer include similar abnormalities seen in the low-grade type plus alterations in the INK4A gene (Cordon-Cardo, 2008). In addition, there can be deletions of 2q, 5q, 10q, 18q, and gains of 5q and 20q (Knowles, 2008b). Alterations of TP21 and TP27 along with TP53 have been reported (Simon et al, 2004). High-grade urothelial carcinoma stains are immunoreactive for cytokeratin-20 and are likely aneuploid.
There are key genetic and phenotypic changes that occur in cancer cells, thus providing the ability to invade the underlying stroma. Invasive urothelial carcinoma is divided into two groups: lamina propria and deep muscle invasion. Lamina propria invasive tumors are high-grade cancers that can be in clusters or in single cells, with single-cell invasion having a worse prognosis (Fig. 80–11). Rarely, low-grade cancers can invade the lamina propria. Vascular invasion can occur within the lamina propria because of the large vascular network within this tissue layer; however, it is frequently overcalled because of retraction artifact around tumor nests. There is a subdivision of lamina propria invasion into T1a (invasion above muscularis mucosa) and T1b (invasion below the muscularis mucosa). This subject will be dealt with more extensively in the Staging section.
Staging
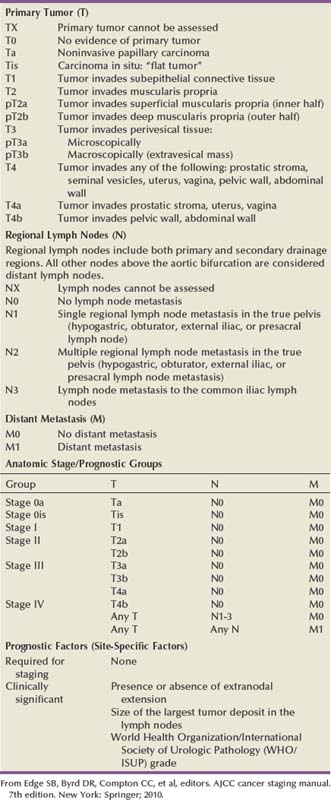
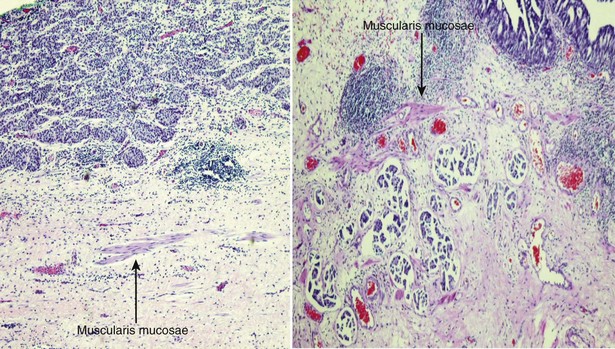
The American Joint Commission on Cancer in combination with the International Union Cancer Consortium meets on a regular basis to determine the tumor, nodes, and metastases (TNM) staging classifications. The 2009 staging system is shown in Table 80–7 (Edge et al, 2010). Ta and CIS disease have no invasion of the basement membrane, but endophytic growth of low-grade tumors into the lamina propria is possible, and cancer can occur in Von Brunn nests (Sung et al, 2006, Jones et al, 2007). T1 disease, as mentioned earlier, can be divided into T1a and T1b disease (Smits et al, 1998). The subdivision is based on the muscularis mucosa, which comprises thin wavy vesicles of muscle within the lamina propria that are associated with large vessels and lymphatics (Fig. 80–12). Some investigators substitute the presence of this large-vessel lymphatic layer for the muscularis mucosa when dividing T1a and T1b disease (Lopez-Beltran and Cheng, 2003). Unfortunately, muscularis mucosa is only identified in 15% to 80% of bladder biopsy specimens and in 90% of radical cystectomy specimens (Cheng et al, 1999; Sozen et al, 2002; Lopez-Beltran and Cheng, 2003; Lopez-Beltran, 2008). The prognostic significance of T1a and T1b disease is inconsistent because of the lack of muscularis mucosa in many bladder biopsy specimens. Essentially, the T1a and T1b stratifications suggest that the deeper the tumor invades in the lamina propria, the worse the survival.
Muscle-invasive disease is subdivided into T2a and T2b. T2a includes invasion into the inner half of the muscularis propria, while T2b is deeper into the outer half. Analysis of the American Joint Committee on Cancer (AJCC) data would suggest that there is a disease-free survival difference between T2a and T2b disease (Edge et al, 2010). On the contrary, Cheng and colleagues (1999b) showed that depth of muscle invasion was not predictive of disease-free survival, but the size of the tumor was the key element. T3 disease constitutes invasion outside the bladder proper into the periadipose tissue. T3a disease involves microscopic extension, while T3b involves macroscopic extension. Clinically, T3a disease is identified by a palpable mass at the time of examination under anesthesia during the initial transurethral resection and subsequently is nonpalpable after the tumor is resected. T3b disease has a persistent palpable mass after transurethral resection of the tumor. Pathologically, T3a disease is microscopic extension into the periadipose tissue, while T3b disease is macroscopic extension. T4a disease is invasion of the prostatic stroma, uterus, or vagina, and T4b disease is invasion of the pelvic wall or abdominal wall. Extension of the tumor into the prostatic urethra without stromal invasion is currently classified under the prostatic urethral section and does not carry an adverse prognosis for patients with known bladder cancer (Pagano et al, 1996; Edge et al, 2010). Prostatic stromal invasion, however, particularly if it is a direct extension from the bladder through the muscle into the prostate, does have a poor prognostic factor, with a 5-year overall survival of less than 25% (Esrig et al, 1996; Pagano et al, 1996).
A major problem in bladder cancer is understaging, occurring in 34% to 64% of cases. Chang and colleagues (2001) reported that 27% of T1 tumors were upstaged after radical cystectomy, and 49% of T2 tumors were upstaged to T3. Because of this understaging, the American Urological Association (AUA) guidelines call for a repeat transurethral resection in patients with T1 tumors to assess for muscle invasive disease even if muscle was present in the specimen (Hall et al, 2007). The significance of this restaging is shown in an article by Herr and colleagues (2007)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree