Bladder Carcinomas
Bladder cancer is the second most common cancer of the genitourinary tract. It accounts for 7% of new cancer cases in men and 2% of new cancer cases in women. The incidence is higher in whites than in African Americans, and there is a positive social class gradient for bladder cancer in both sexes. The average age at diagnosis is 65 years. At that time, approximately 75% of bladder cancers are localized to the bladder; 25% have spread to regional lymph nodes or distant sites.
Cigarette smoking accounts for 65% of cases in men and 20–30% in women. In general, smokers have approximately a two- to threefold increased risk of bladder cancer than nonsmokers, and the association appears to be dose related. The causative agents are thought to be alpha- and beta-naphthylamine, which are secreted into the urine of smokers. The risk of bladder appears to decrease after smoking cessation but may not reach the levels of never smokers.
Occupational exposure accounts for 15–35% of cases in men and 1–6% in women (Matanoski and Elliott, 1981). Workers in the chemical, dye, rubber, petroleum, leather, and printing industries are at increased risk. Specific occupational carcinogens include benzidine, beta-naphthylamine, and 4-aminobiphenyl, and the latency period between exposure and tumor development may be prolonged. Patients who have received cyclophosphamide (Cytoxan) for the management of various malignant diseases are also at increased risk (Fairchild et al, 1979). Ingestion of artificial sweeteners has been proposed to be a risk factor, but several studies have failed to confirm any association (Elcock and Morgan, 1993). Physical trauma to the urothelium induced by infection, instrumentation, and calculi increases the risk of malignancy (Hicks, 1982).
The exact genetic events leading to the development of bladder cancer are unknown, but they are likely to be multiple and may involve the activation of oncogenes and inactivation or loss of tumor suppressor genes (Olumi et al, 1990). Loss of genetic material on chromosome 9 appears to be a consistent finding in patients with both low-grade, low-stage and high-grade, high-stage disease (Miyao et al, 1993; Tsai et al, 1990), which suggests that this may be an early event in bladder cancer development. Loss of chromosome 9 in multiple tumors from an individual patient supports the concept that genetic changes in bladder cancer represent a “field defect” that may occur throughout the urothelium. More recent studies examining p53 tumor suppressor gene mutations in primary, recurrent, and upper-tract tumors suggest that these tumors can have a single clonal origin (Dalbagni et al, 2001; Sidransky et al, 1991). Additional genetic changes have been described that are specific for invasive bladder tumors. Chromosome 11p, which contains the c-Ha-ras proto-oncogene, is deleted in approximately 40% of bladder cancers (Olumi et al, 1990). Increased expression of the c-Ha-ras protein product, p21, has been detected in dysplastic and high-grade tumors but not in low-grade bladder cancers. Deletions of chromosome 17p have also been detected in over 60% of all invasive bladder cancers, but 17p deletions have not been described in superficial tumors. This finding is noteworthy because the p53 tumor suppressor gene maps to chromosome 17p. TP53 alterations represent the most commonly identified genetic abnormality in human cancers, making deletion of this chromosome an important finding in carcinoma in situ (CIS) and muscle invasive bladder cancer. Mutations of the fibroblast growth factor receptor 3 (FGFr3) are found in >60% of papillomas and low-grade bladder tumors and hence it is considered an oncogene. Ras mutations are also found in both low- and high-grade or muscle invasive tumors but Ras and FGFr3 mutations appear to be mutually exclusive (Jebar et al, 2005) and both are involved in activation of the MAP Kinase pathway. p53 mutations are less common in low-grade tumors and loss of FGFr3 with increased expression of p53 has been associated with higher stage and grade (Knowles, 2007).
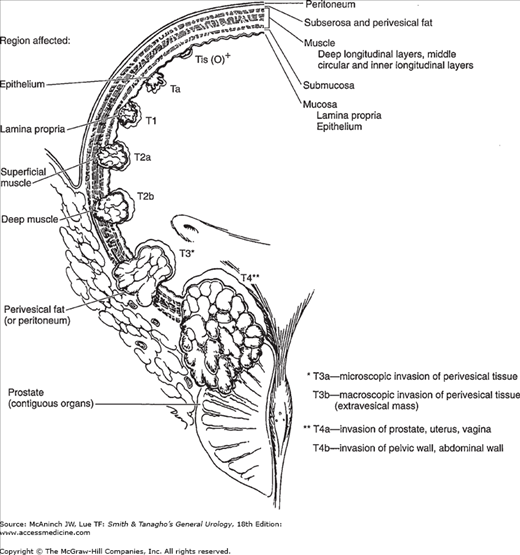
Currently, the most commonly used staging system allows for a precise and simultaneous description of the primary tumor stage (T stage), the status of lymph nodes (N stage), and metastatic sites (M stage) (American Joint Committee on Cancer, 1997). The T staging system is depicted in Figure 21–1. Nodal (N) stage is defined as Nx—cannot be assessed, N0—no nodal metastases, N1—single node <2 cm involved, N2—single node involved 2–5 cm in size or multiple nodes none >5 cm, N3—one or more nodes >5 cm in size involved. Metastases (M) stage is defined as Mx—cannot be defined, M0—no distant metastases, M1—distant metastases present. Staging errors exist when one compares the clinical stage (that based on physical examination and imaging) with the pathologic stage (that based on removal of the bladder and regional lymph nodes). Overstaging is relatively uncommon, but clinical understaging may occur in up to 53% of patients (Dutta et al, 2001; Skinner, 1982).
Ninety-eight percent of all bladder cancers are epithelial malignancies, with the predominant majority being transitional cell carcinomas (TCCs). About 5% are adenocarcinomas or squamous cell carcinomas.
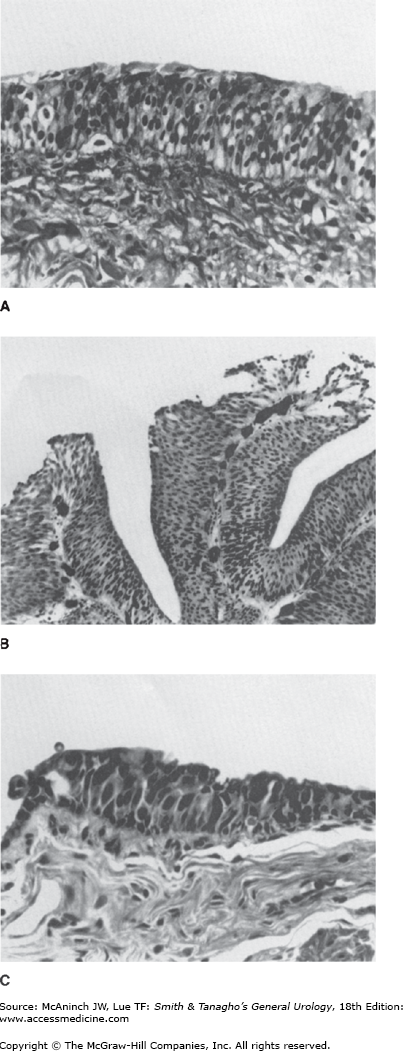
The normal urothelium is composed of 3–7 layers of transitional cell epithelium resting on a basement membrane composed of extracellular matrix (collagen, adhesive glycoproteins, glycosaminoglycans) (Figure 21–2A). The epithelial cells vary in appearance: The basal cells are actively proliferating cells resting on the basement membrane; the luminal cells, perhaps the most important feature of normal bladder epithelium, are larger umbrella-like cells that are bound together by tight junctions. Beyond the basement membrane is loose connective tissue, the lamina propria, in which occasionally smooth-muscle fibers can be identified. These fibers should be distinguished from deeper, more extensive muscle elements defining the true muscularis propria.
The World Health Organization recognizes a papilloma as a papillary tumor with a fine fibrovascular stalk supporting an epithelial layer of transitional cells with normal thickness and cytology (Epstein et al, 1998). These are also termed papillary urothelial neoplasms of low malignant potential or PUNLMP. PUNLMPs are a rare benign condition that do not require aggressive therapy.
Approximately 90% of all bladder cancers are TCCs. These tumors most commonly appear as papillary, exophytic lesions (Figure 21–2B); less commonly, they may be sessile or ulcerated. Whereas the former group is usually superficial in nature, sessile growths are often invasive.
CIS is recognizable as flat, anaplastic epithelium. The urothelium lacks the normal cellular polarity, and cells contain large, irregular hyperchromatic nuclei with prominent nucleoli (Figure 21–2C).
Adenocarcinomas account for <2% of all bladder cancers. Primary adenocarcinomas of the bladder may be preceded by cystitis and metaplasia. Histologically, adenocarcinomas are mucus secreting and may have glandular, colloid, or signet-ring patterns. Whereas primary adenocarcinomas often arise along the floor of the bladder, adenocarcinomas arising from the urachus occur at the dome. Both tumor types are often localized at the time of diagnosis, but muscle invasion is usually present. Five-year survival is usually <40%, despite aggressive surgical management (Abenoza et al, 1987; Bernstein et al, 1988; Kramer et al, 1979).
Squamous cell carcinoma accounts for between 5% and 10% of all bladder cancers in the United States and is often associated here with a history of chronic infection, vesical calculi, or chronic catheter use. It may also be associated with bilharzial infection owing to Schistosoma haematobium, because squamous cell carcinoma accounts for approximately 60% of all bladder cancers in Egypt, parts of Africa, and the Middle East, where this infection is prevalent (El-Bolkainy et al, 1981). These tumors are often nodular and invasive at the time of diagnosis. Histologically they appear as poorly differentiated neoplasms composed of polygonal cells with characteristic intercellular bridges. Keratinizing epithelium is present, although often in small amounts.
Undifferentiated bladder carcinomas, which are rare (accounting for <2%), have no mature epithelial elements. Very undifferentiated tumors with neuroendocrine features and small cell carcinomas tend to be aggressive and present with metastases (Choong et al, 2005; Quek et al, 2005).
Mixed carcinomas constitute 4–6% of all bladder cancers and are composed of a combination of transitional, glandular, squamous, or undifferentiated patterns. The most common type comprises transitional and squamous cell elements (Murphy, 1989). Most mixed carcinomas are large and infiltrating at the time of diagnosis.
Rare epithelial carcinomas identified in the bladder include villous adenomas, carcinoid tumors, carcinosarcomas, and melanomas. Rare nonepithelial cancers of the urinary bladder include pheochromocytomas, lymphomas, choriocarcinomas, and various mesenchymal tumors (hemangioma, osteogenic sarcoma, and myosarcoma) (Murphy, 1989). Cancers of the prostate, cervix, and rectum may involve the bladder by direct extension. The most common tumors metastatic to the bladder include (in order of incidence) melanoma, lymphoma, stomach, breast, kidney, lung, and liver (Franks et al, 1999; Goldstein, 1967; Murphy, 1989).
Hematuria is the presenting symptom in 85–90% of patients with bladder cancer. It may be gross or microscopic, intermittent rather than constant. In a smaller percentage of patients, it is accompanied by symptoms of vesical irritability: frequency, urgency, and dysuria. Irritative voiding symptoms seem to be more common in patients with diffuse CIS. Symptoms of advanced disease include bone pain from bone metastases or flank pain from retroperitoneal metastases or ureteral obstruction.
Patients with large-volume or invasive tumors may be found to have bladder wall thickening or a palpable mass—findings that may be detected on a careful bimanual examination under anesthesia. If the bladder is not mobile, that suggests fixation of tumor to adjacent structures by direct invasion.
Hepatomegaly and supraclavicular lymphadenopathy are signs of metastatic disease. Lymphedema from occlusive pelvic lymphadenopathy may be seen occasionally. Patients may also present with back pain or pathologic fracture from bony metastases. On rare occasions, metastases can occur in unusual sites such as the skin presenting as painful nodules with ulceration (Block et al, 2006).
The most common laboratory abnormality is hematuria. It may be accompanied by pyuria, which on occasion may result from concomitant urinary tract infection. Azotemia may be noted in patients with ureteral occlusion owing to the primary bladder tumor or lymphadenopathy. Anemia may be a presenting symptom owing to chronic blood loss, or replacement of the bone marrow with metastatic disease.
Exfoliated cells from both normal and neoplastic urothelium can be readily identified in voided urine. Larger quantities of cells can be obtained by gently irrigating the bladder with isotonic saline solution through a catheter or cystoscope (barbotage). Cytologic examination of exfoliated cells may be especially useful in detecting cancer in symptomatic patients and assessing response to treatment. Detection rates are high for tumors of high grade and stage as well as CIS but not as impressive for low-grade superficial tumors.
Several new tests have been developed in order to overcome the shortcomings of urinary cytology such as the low sensitivity for low-grade superficial tumors and inter-observer variability. Commercially available tests include, the bladder tumor antigen (BTA) stat test (Bard Diagnostic Sciences, Inc, Redmond, WA), the BTA TRAK assay (Bard Diagnostic Sciences, Inc), NMP22 assay, and the NMP22 BladderChek test, ImmunoCyt and UroVysion. These tests can detect cancer specific proteins in urine (BTA/NMP22) or augment cytology by identifying cell surface or cytogenetic markers in the nucleus (UroVysion and ImmunoCyt). Other tests under investigation include identification of the Lewis X antigen on exfoliated urothelial cells, and the determination of telomerase activity in exfoliated cells. Several studies have examined the performance of these voided urinary markers for the detection and follow-up of patients with bladder cancer (Grossfeld et al, 2001; Konety and Getzenberg, 2001) (Table 21–1).
| Marker | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
Cytology | 35–61 | 93–100 | — | — |
NMP22 | 49–68 | 86–88 | 29–65 | 60–100 |
BTA stat | 57–83 | 68–85 | 20–56 | 70–95 |
BTA TRAK | 54–91 | 28–84 | 62 | 73 |
Telomerase | 62–80 | 60–99 | 84 | 89 |
UroVysion | 30–72 | 63–95 | 45–92 | 31–88 |
ImmunoCyt | 76–85 | 63–75 | 29–63 | 81–96 |
Cytokeratin 20 | 91 | 85 | 95 | 76 |
These tests have been demonstrated to enhance detection of bladder cancer when used either individually or in combination with cytology. They have been used to detect both new index tumors as well as recurrent tumors. Some of the protein markers lack the specificity of cytology thereby hampering their widespread use. Studies are currently evaluating the precise role of these urinary markers in bladder cancer and their optimal role in the diagnosis and surveillance of bladder cancer is still being determined.
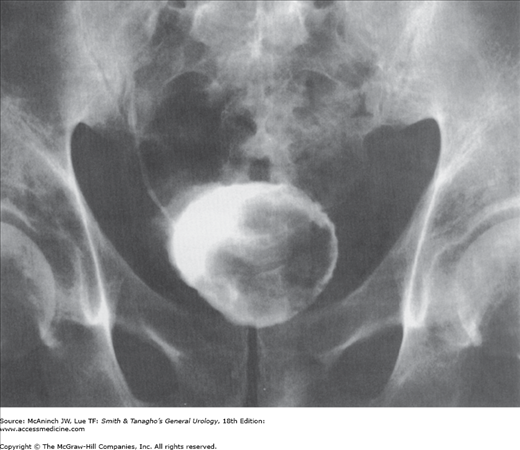
Although bladder cancers may be detected by various imaging techniques, their presence is confirmed by cystoscopy and biopsy. Imaging is therefore used to evaluate the upper urinary tract and, when infiltrating bladder tumors are detected, to assess the depth of muscle wall infiltration and the presence of regional or distant metastases. Intravenous urography used to be one of the most common imaging tests for the evaluation of hematuria. However, it has virtually been replaced by computed tomography (CT) urography, which is more accurate, for evaluation of the entire abdominal cavity, renal parenchyma, and ureters in patients with hematuria (Gray Sears et al, 2002). Bladder tumors may be recognized as pedunculated, radiolucent filling defects projecting into the lumen (Figure 21–3); nonpapillary, infiltrating tumors may result in fixation or flattening of the bladder wall. Hydronephrosis from ureteral obstruction is usually associated with deeply infiltrating lesions and poor outcome after treatment (Haleblian et al, 1998).
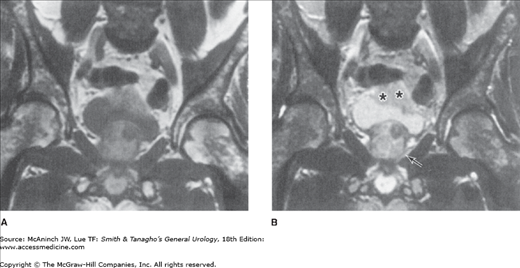
Superficial (Ta, Tis) bladder cancers staged with a properly performed TUR and examination under anesthesia do not require additional imaging of the bladder or pelvic organs. However, higher stage lesions are often understaged, and the addition of imaging may be useful. Both CT and magnetic resonance imaging (MRI) (Figure 21–4) have been used to characterize the extent of bladder wall invasion and detect enlarged pelvic lymph nodes, with overall staging accuracy ranging from 40% to 85% for CT and from 50% to 90% for MRI (Fisher et al, 1985; Wood et al, 1988). Both techniques rely on size criteria for the detection of lymphadenopathy: lymph nodes >1 cm are thought to be suggestive of metastases; unfortunately, small-volume pelvic lymph node metastases are often missed. Because invasive bladder cancers may metastasize to the lung or bones, staging of advanced lesions is completed with chest x-ray and radionuclide bone scan. Bone scans can be avoided if the serum alkaline phosphatase is normal (Berger, 1981). More recently positron emission tomography (PET) CT scans have been utilized to assess metastases from bladder cancer. Early data suggests that PET CT may be able to detect microscopic metastases in lymph nodes that otherwise appear normal with a sensitivity of 70% and specificity of 94% (Kibel et al, 2009). However it is still unclear if such information does lead to significant change in management.
Figure 21–4.
MRI scan of invasive bladder carcinoma: A: T1-weighted image; B: T2-weighted image. Bladder wall invasion is best assessed on T2-weighted images because of heightened contrast between tumor (asterisks) and detrusor muscle along with ability to detect interruption of the thin high-intensity line representing normal bladder wall. The heterogeneous appearance of the prostate (arrow) on the T2-weighted image owes to benign prostatic hypertrophy, confirmed at cystectomy. MRI, magnetic resonance imaging.
The diagnosis and initial staging of bladder cancer is made by cystoscopy and transurethral resection (TUR). Cystoscopy can be done with either flexible or rigid instruments, although the former is associated with less discomfort and only requires local anesthesia. Superficial, low-grade tumors usually appear as single or multiple papillary lesions. Higher-grade lesions are larger and sessile. CIS may appear as flat areas of erythema and mucosal irregularity. Use of fluorescent cystoscopy with blue light can enhance the ability to detect lesions by as much as 20% (Jocham et al, 2005). In this procedure, hematoporphyrin derivatives that accumulate preferentially in cancer cells are instilled into the bladder and fluorescence incited using a blue light. Cancer cells with accumulated porphyrin such as 5-aminolevulinic acid or hexaminolevulinate (HAL) are detected as glowing red under the fluorescent light (Loidl et al, 2005). This technology can be particularly useful in the detection of CIS.
Once a tumor is visualized or suspected, the patient is scheduled for examination under anesthesia and TUR or biopsy of the suspicious lesion. The objectives are tumor diagnosis, assessment of the degree of bladder wall invasion (staging), and complete excision of the low-stage lesions amenable to such treatment. The American Urologic Associations’ best practice guidelines for bladder cancer state that as a standard, all patients undergo as complete a resection as possible of all visible tumors (Hall et al, 2007).
Patients are placed in the lithotomy position. A careful bimanual examination is performed. The presence of any palpable mass and mobility of the bladder are noted along with any degree of fixation to contiguous structures. Cystoscopy is repeated with one or more lenses (30° and 70°) that permit complete visualization of the entire bladder surface. A resectoscope is then placed into the bladder, and visible tumors are removed by electrocautery. Suspicious areas may be biopsied with cup biopsy forceps and the areas may be cauterized with an electrode. Some clinicians routinely perform random bladder biopsies of normal-appearing urothelium both close to and remote from the tumor. The value of random bladder biopsies is controversial. Detection of CIS on these biopsies can alter treatment though more recent studies suggest that only 1.5% of low-risk and 3.5% of high-risk patients may have tumor detected on such biopsies (van der Meijden et al, 1999; May et al, 2003). Findings of the random biopsy can alter treatment in up to 7% of patients (May et al, 2003). Using fluorescent cystoscopy may allow for more precise assessment of the completeness of tumor resection, thereby reducing the risk of leaving behind unresected tumor.
The natural history of bladder cancers is defined by two separate but related processes: tumor recurrence and progression. Progression, including metastasis, represents the greater biologic risk. However, recurrence, even without progression, represents substantial patient morbidity in that it requires periodic reevaluation (cytology, cystoscopy, etc), repeat endoscopic ablation, and often intravesical chemotherapy (which may be costly, uncomfortable, and associated with complications). Treatment decisions are based on tumor stage and grade. Staging is performed using the tumor, node, metastasis (TNM) staging system (Figure 21–1; Table 21–2) while grading has changed from the Ash-Broder system (I–III or I–IV). The new WHO-ISUP system segregates tumors into papillary urothelial neoplasm of low malignant potential (PUNLMP), low grade or high grade.
Cancer stage | Initial treatment options |
|---|---|
Tis | Complete TUR followed by intravesical BCG |
Ta (single, low-to-moderate grade, not recurrent) | Complete TUR |
Ta (large, multiple, high grade, or recurrent) | Complete TUR followed by intravesical chemo- or immunotherapy |
T1 | Complete TUR followed by intravesical chemo- or immunotherapy or radical cystectomy |
T2–T4 | Radical cystectomy Neoadjuvant chemotherapy followed by radical cystectomy Radical cystectomy followed by adjuvant chemotherapy Concomitant chemotherapy and irradiation |
Any T, N+, M+ | Systemic chemotherapy followed by selective surgery or irradiation |
At initial presentation, 74% of bladder tumors are superficial or nonmuscle invasive—stage Tis, Ta, or T1 (David et al, 2009). Invasion into the muscle wall and beyond is identified in a smaller number of patients, approximately 26; regional or distant metastases are found in approximately 25%. Unfortunately, 80% of patients with invasive or metastatic disease have no previous history of bladder cancer (Kaye and Lange, 1982). About 47% of the tumors are high grade and 53% are low grade at diagnosis (David et al, 2009). A majority of patients with T1 disease can be further subclassified into groups based on the level of lamina propria invasion. The depth of lamina propria invasion is predictive of the likelihood of recurrence and progression (Orsola et al 2005). There are strong correlations between tumor grade and stage and tumor recurrence, progression, and survival (Frazier et al, 1993). Patients with low-stage, low-grade disease have a low risk (<5%) of progression to invasive disease, while as many as 40% of patients with low-stage but high-grade disease will progress with extended follow-up (Herr, 2000). Disease-free survival is excellent for patients with pathologically confirmed superficial disease (pT0, pT1, pTIS, 80–88%). However, it falls for patients with pT2 (53–80%), pT3 (39–68%), and pT4 (25–40%) tumors (Frazier et al, 1993; Stein et al, 2001; Thrasher et al, 1994)—owing to the greater likelihood of metastasis in tumors of higher stage. Although lymph node metastases are uncommon (5%) in tumors of low stage, they are increasingly more common in higher stage tumors: 10–30% for pT3A, 31–46% for pT3B, and 35–64% for pT4 (Frazier et al, 1993; Stein et al, 2001). In patients with organ-confined disease, the presence of pelvic lymph node metastases appears to be the most important prognostic factor (Vieweg et al, 1999). The presence of lymphovascular invasion even in those with node-negative disease may portend a worse prognosis (Lotan et al, 2005).
Although metastasis is less common with superficial bladder cancers, such tumors may progress; most recur and require additional treatment. Tumor progression occurs in <6% of patients with Ta disease, but in up to 53% of those with T1 disease, with or without concomitant CIS (Cookson et al, 1997; Heney et al, 1983). Tumor progression occurs in 10–20% of patients with grade I tumors, 19–37% with grade II tumors, and 33–64% with grade III tumors (Lutzeyer et al, 1982; Torti et al, 1987). Using the more recent grading system, progression is observed in 5% of those with low-grade tumors, 15–40% with high-grade tumors, while PUNLMPs almost never demonstrate any risk of progression (Epstein et al, 1998).
Tumor recurrence is related to history of disease and grade, number, and size of the tumor. It is more common in the first 12–24 months after diagnosis (but can become manifest many years later), and patients with one recurrence are more likely to have another. Patients with T1, multiple (>4), large (>3), or high-grade tumors are at greater risk, as are those with either CIS or severe dysplasia in normal-appearing urothelium remote from the tumor site (Heney et al, 1983; Wolf et al, 1985). Tumors can be stratified into low-, intermediate- and high-risk categories based on these criteria and this can be used to guide management decisions.
Conventional histopathologic analysis of bladder tumors, including determination of tumor grade and stage, may not reliably predict the behavior of many bladder cancers. Assessment of molecular markers of disease, with immunohistochemical methods, in biopsy specimens or cystectomy specimens can yield useful prognostic information.
Tumor growth and metastasis require the growth of new blood vessels, through angiogenesis. Angiogenic stimulators, such as the fibroblastic growth factors and vascular endothelial growth factor, and angiogenic inhibitors, such as thrombospondin-1 and angiostatin regulate angiogenesis. Immunohistochemical quantification of angiogenesis in a given tumor by measuring microvessel density is a useful prognostic indicator for a variety of human malignancies, including bladder cancer. In bladder cancer, microvessel density has been associated with lymph node metastases, disease progression, and overall survival of patients with invasive bladder cancer treated with radical cystectomy (Bochner et al, 1997; Dickinson et al, 1994; Jaeger et al, 1995). The p53 gene is a tumor suppressor gene that plays a key role in the regulation of the cell cycle. When deoxyribonucleic acid (DNA) damage occurs, the level of p53 protein increases, causing cell cycle arrest and repair of DNA. Mutations in the p53 gene result in the production of an abnormal protein product, allowing cells with damaged DNA to continue through the cell cycle. The altered p53 protein has a prolonged half-life compared with the wild-type protein, allowing for its detection by immunohistochemical techniques. Patients with altered p53 expression (indicating possible mutation of the p53 gene) appear to have an increased risk for disease recurrence and a decreased overall survival when compared with patients with normal p53 expression (Esrig et al, 1995). Cancers that are p53 positive are associated with recurrence rates of 62% for pT1, 56% for pT2, and 80% for pT3a, compared with 7%, 12%, and 11%, respectively, for cancers without p53 reactivity.
Alteration of the retinoblastoma (Rb) gene, a tumor suppressor gene, is associated with high-grade, high-stage bladder cancers. In addition, Rb alteration appears to be significantly associated with decreased overall survival in such patients (Cordon-Cardo et al, 1992; Logothetis et al, 1992). Studies in which both p53 and Rb have been examined in patients with invasive bladder cancer suggest that bladder tumors with alterations in both genes have a poorer prognosis and decreased overall survival when compared with tumors with wild-type p53 and Rb.
Assessment of other markers that may correlate with outcome in patients with bladder cancer includes that of tumor growth fraction (proliferative index) and cellular adhesion molecule expression (E-cadherin) (Lipponen and Eskelinen, 1995; Okamura et al, 1990).
Patients with superficial bladder cancers can be treated with TUR followed by selective intravesical chemotherapy or immunotherapy. Patients with initial low-grade small tumors are at low risk of progression and may be treated by TUR alone followed by surveillance or intravesical chemotherapy. In some patients with recurrent, low-grade tumors, fulguration of such tumors using electrocautery in an office setting under local anesthesia is also an acceptable alternative. Recent data suggest that some low-grade tumors can be observed at least for a period of time without significant increased risk of progression or metastases (Soloway et al, 2003). Patients with T1, high-grade, multiple, large, recurrent tumors or those associated with CIS on bladder biopsies are at a higher risk of progression and recurrence and should be considered candidates for intravesical chemotherapy or immunotherapy after complete and careful TUR. A second resection of the same area may be required to accurately stage disease and determine treatment (Herr et al, 1999; Grimm et al, 2003). Repeat resections may also enhance response to intravesical therapy (Herr, 2005). Management of T1 tumors is somewhat controversial; some clinicians advise radical cystectomy, especially for high-grade lesions, which are associated with a high rate of progression. However, progression rates can be reduced by intravesical immunotherapy (Cookson and Sarosdy, 1992; Herr et al, 1989). Recurrence of T1 disease after a trial of intravesical therapy warrants more aggressive therapy such as cystectomy (Herr, 1991; Herr and Sogani, 2001).
Patients with more invasive, but still localized, tumors (T2, T3) are candidates for more aggressive local treatment, including partial or radical cystectomy, or a combination of radiation and systemic chemotherapy. Radical TUR alone may be a viable option in select patients with T2 disease, particularly if no tumor is found on repeat resection since 10-year survival rates as high as 83% can be achieved (Herr, 2001). However, this approach must be used with caution since there is a substantial risk of leaving residual disease behind (Solsona et al, 1998). Superficial ductal or acinar in situ carcinoma of the prostatic urethra, not invading the basement membrane or prostatic stroma, may be treated with TUR and intravesical chemotherapy or immunotherapy rather than cystectomy. However, patients with more extensive involvement of the prostatic urethra by TCC, or recurrence after conservative therapy, require more aggressive therapy. Patients with unresectable local tumors (T4B) are candidates for systemic chemotherapy, followed by surgery (or possibly irradiation). Patients with either local or distant metastases should receive systemic chemotherapy followed by the selective use of either irradiation or surgery, depending on the response.
Immunotherapeutic or chemotherapeutic agents can be instilled into the bladder directly via catheter, thereby avoiding the morbidity of systemic administration in most cases. Intravesical therapy can have a prophylactic or therapeutic objective, either to reduce recurrence in patients whose tumors have been completely resected. Intravesical chemotherapy is used in two settings. When instilled immediately following TUR, it acts prophylactically to reduce tumor cell implantation (Solsona et al, 1999). It can also be used therapeutically to reduce risk of recurrence and progression particularly for low-risk superficial tumors. Therefore, intravesical chemotherapy or immunotherapy may be delivered in three different fashions to achieve individual goals (Table 21–3). Considerable experience has been gained, but comparison of different agents is difficult owing to the paucity of randomized trials and variations in dose, contact time, patient population, and intervals between treatments. Most agents are administered weekly for 6 weeks except when being used prophylactically where a single dose is administered immediately following TUR. Maintenance therapy (ie, monthly or bimonthly intravesical therapy) may decrease recurrence rates further. Although local toxicity is relatively common—primarily irritative voiding symptoms—systemic toxicity is rare because of the limited absorption of drugs across the lumen of the bladder. Severe systemic complications can be avoided by not administering intravesical chemotherapy in patients with gross hematuria. Efficacy may be improved by increasing contact time and drug concentration (ie, by restricting fluid intake before administration, asking the patient to lie in different positions during treatment, avoiding instillation of air during drug administration, and requiring the patient to avoid urinating for 1–2 hours thereafter). The most common agents in the United States are mitomycin C, thiotepa, and Bacillus Calmette-Guérin (BCG). Patients in whom treatment with one agent fails may respond to another.
Use | Timing | Goal |
|---|---|---|
Adjunctive | At TUR | Prevent implantation |
Prophylactic | After complete TUR | Prevent or delay recurrence or progression |
Therapeutic | After incomplete TUR | Cure residual disease |
Mitomycin C is an antitumor, antibiotic, alkylating agent that inhibits DNA synthesis. With a molecular weight of 329, systemic absorption is minimal. The usual dose is 40 mg in 40 cc of sterile water or saline given once a week for 6 weeks. The same dose is utilized for a single prophylactic instillation. Between 39% and 78% of patients with residual tumor experience, a complete response to intravesical mitomycin C (Kowalkowski and Lamm, 1988) and recurrence is reduced in 2–33% after complete TUR (Herr et al, 1987). Side effects are noted in 10–43% of patients and consist largely of irritative voiding symptoms including urinary frequency, urgency, and dysuria. Unique to this drug is the appearance of a rash on the palms and genitalia in approximately 6% of patients, but this effect can be alleviated if patients wash their hands and genitalia at the time of voiding after intravesical administration. Instillation of Mitomycin C into the bladder immediately post-TUR has been shown to decrease recurrences and prolong the interval to recurrence (Sylvester et al, 2004). Hence it is now considered standard of care to instill one dose of 40 mg of Mitomycin C into the bladder immediately post-TUR to reduce risk of recurrence (Hall et al, 2007). The efficacy of Mitomycin C can be enhanced by administering it in a more concentrated solution of 40 mg in 20 cc of sterile water after alkalinizing the urine and with reduced fluid intake (Au et al, 2001).
Thiotepa is an alkylating agent with a molecular weight of 189. Although various doses have been used, 30 mg weekly seems to be sufficient. Up to 55% of patients respond completely. Most series show significantly lower recurrence rates in patients taking thiotepa than in those taking a placebo (Herr et al, 1987; Kowalkowski and Lamm, 1988). Cystitis is not uncommon after instillation but is usually mild and self-limited. Myelosuppression manifested as leukopenia and thrombocytopenia occurs in up to 9% of patients owing to systemic absorption. A complete blood count should be obtained in all patients before successive instillations.
BCG is an attenuated strain of Mycobacterium bovis. Many different strains of BCG exist, and the marketed preparations vary in the number, pathogenicity, viability, and immunogenicity of organisms (Catalona and Ratliff, 1990). The exact mechanism by which BCG exerts its antitumor effect is unknown, but it seems to be immunologically mediated. Mucosal ulceration and granuloma formation are commonly seen after intravesical instillation. Activated helper T lymphocytes can be identified in the granulomas, and interleukin-2 reportedly can be detected in the urine of treated patients (Haaf et al, 1986). BCG has been shown to be very effective both therapeutically and prophylactically. It appears to be the most efficacious intravesical agent for the management of CIS. Complete responses are recorded in 36–71% of patients with residual carcinoma (Catalona and Ratliff, 1990; Herr et al, 1987). Recurrence rates are reduced substantially in patients treated after endoscopic resection (11–27% versus a 70% recurrence after endoscopic resection alone) (Catalona and Ratliff, 1990; Herr et al, 1985; Herr et al, 1987; Lamm, 1985). BCG has been shown to be superior to intravesical chemotherapy in preventing recurrence in patients with high-risk superficial bladder cancer (Lamm et al, 1991). Although BCG appears to be effective in delaying progression of high-risk superficial bladder cancer, 40–50% of these patients will experience disease progression with extended follow-up and many patients will ultimately require cystectomy (Cookson et al, 1997; Davis et al, 2002; Herr et al, 1995). The most commonly recommended induction regimen for BCG is weekly for 6 weeks followed by a period of 6 weeks where no BCG is given. Maintenance therapy should be considered in high-risk patients (Lamm et al, 2000). The optimal regimen for maintenance therapy is unclear. Published regimens involve three instillations once a week at 3- to 6-month intervals for 3 years following TUR. Only a small proportion (16–32%) of patients received all the treatments in prior studies, which highlights the difficulty of administering maintenance therapy and its side effects (van der Meijden et al, 2003; Lamm et al, 2000
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree