Embryology of the Genitourinary System: Introduction
At birth, the genital and urinary systems are related only in the sense that they share certain common passages. Embryologically, however, they are intimately related. Because of the complex interrelationships of the embryonic phases of the two systems, they are discussed here as five subdivisions: the nephric system, the vesicourethral unit, the gonads, the genital duct system, and the external genitalia.
Nephric System
The nephric system develops progressively as three distinct entities: pronephros, mesonephros, and metanephros.
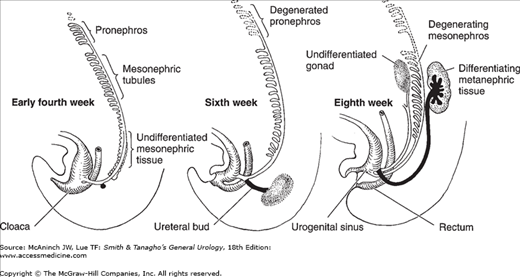
The pronephros is the earliest nephric stage in humans, and it corresponds to the mature structure of the most primitive vertebrate. It extends from the 4th to the 14th somites and consists of six to ten pairs of tubules. These open into a pair of primary ducts that are formed at the same level, extend caudally, and eventually reach and open into the cloaca. The pronephros is a vestigial structure that disappears completely by the 4th week of embryonic life (Figure 2–1).
Figure 2–1.
Schematic representation of the development of the nephric system. Only a few of the tubules of the pronephros are seen early in the 4th week, while the mesonephric tissue differentiates into mesonephric tubules that progressively join the mesonephric duct. The first sign of the ureteral bud from the mesonephric duct is seen. At 6 weeks, the pronephros has completely degenerated and the mesonephric tubules start to do so. The ureteral bud grows dorsocranially and has met the metanephrogenic cap. At the 8th week, there is cranial migration of the differentiating metanephros. The cranial end of the ureteric bud expands and starts to show multiple successive outgrowths. (Adapted from several sources.)
The mature excretory organ of the larger fish and amphibians corresponds to the embryonic mesonephros. It is the principal excretory organ during early embryonic life (4–8 weeks). It too gradually degenerates, although parts of its duct system become associated with the male reproductive organs. The mesonephric tubules develop from the intermediate mesoderm caudal to the pronephros shortly before pronephric degeneration. The mesonephric tubules differ from those of the pronephros in that they develop a cuplike outgrowth into which a knot of capillaries is pushed. This is called Bowman’s capsule, and the tuft of capillaries is called a glomerulus. In their growth, the mesonephric tubules extend toward and establish a connection with the nearby primary nephric duct as it grows caudally to join the cloaca (Figure 2–1). This primary nephric duct is now called the mesonephric duct. After establishing their connection with the nephric duct, the primordial tubules elongate and become S-shaped. As the tubules elongate, a series of secondary branchings increase their surface exposure, thereby enhancing their capacity for interchanging material with the blood in adjacent capillaries. Leaving the glomerulus, the blood is carried by one or more efferent vessels that soon break up into a rich capillary plexus closely related to the mesonephric tubules. The mesonephros, which forms early in the 4th week, reaches its maximum size by the end of the 2nd month.
The metanephros, the final phase of development of the nephric system, originates from both the intermediate mesoderm and the mesonephric duct. Development begins in the 5- to 6-mm embryo with a budlike outgrowth from the mesonephric duct as it bends to join the cloaca. This ureteral bud grows cephalad and collects mesoderm from the nephrogenic cord of the intermediate mesoderm around its tip. This mesoderm with the meta-nephric cap moves, with the growing ureteral bud, more and more cephalad from its point of origin. During this cephalic migration, the metanephric cap becomes progressively larger, and rapid internal differentiation takes place. Meanwhile, the cephalic end of the ureteral bud expands within the growing mass of metanephrogenic tissue to form the renal pelvis (Figure 2–1). Numerous outgrowths from the renal pelvic dilatation push radially into this growing mass and form hollow ducts that branch and rebranch as they push toward the periphery. These form the primary collecting ducts of the kidney. Mesodermal cells become arranged in small vesicular masses that lie close to the blind end of the collecting ducts. Each of these vesicular masses will form a uriniferous tubule draining into the duct nearest to its point of origin.
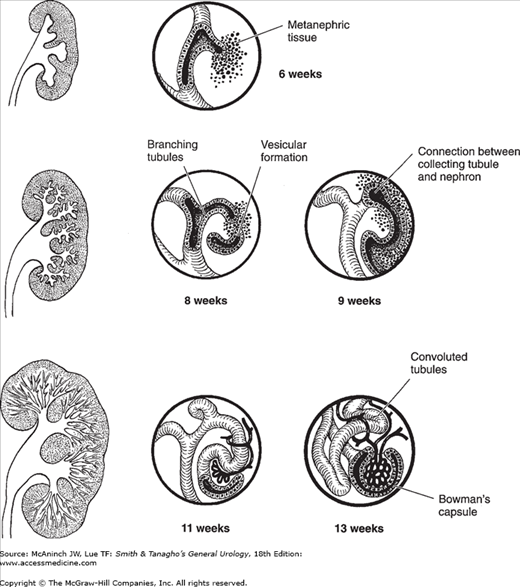
As the kidney grows, increasing numbers of tubules are formed in its peripheral zone. These vesicular masses develop a central cavity and become S-shaped. One end of the S coalesces with the terminal portion of the collecting tubules, resulting in a continuous canal. The proximal portion of the S develops into the distal and proximal convoluted tubules and into Henle’s loop; the distal end becomes the glomerulus and Bowman’s capsule. At this stage, the undifferentiated mesoderm and the immature glomeruli are readily visible on microscopic examination (Figure 2–2). The glomeruli are fully developed by the 36th week or when the fetus weighs 2500 g (Osathanondh and Potter, 1964a and 1964b). The metanephros arises opposite the 28th somite (fourth lumbar segment). At term, it has ascended to the level of the first lumbar or even the twelfth thoracic vertebra. This ascent of the kidney is due not only to actual cephalic migration but also to differential growth in the caudal part of the body. During the early period of ascent (7th to 9th weeks), the kidney slides above the arterial bifurcation and rotates 90°. Its convex border is now directed laterally, not dorsally. Ascent proceeds more slowly until the kidney reaches its final position.
Figure 2–2.
Progressive stages in the differentiation of the nephrons and their linkage with the branching collecting tubules. A small lump of metanephric tissue is associated with each terminal collecting tubule. These are then arranged in vesicular masses that later differentiate into a uriniferous tubule draining into the duct near which it arises. At one end, Bowman’s capsule and the glomerulus differentiate; the other end establishes communication with the nearby collecting tubules.
Certain features of these three phases of development must be emphasized: (1) The three successive units of the system develop from the intermediate mesoderm. (2) The tubules at all levels appear as independent primordia and only secondarily unite with the duct system. (3) The nephric duct is laid down as the duct of the pronephros and develops from the union of the ends of the anterior pronephric tubules. (4) This pronephric duct serves subsequently as the mesonephric duct and as such gives rise to the ureter. (5) The nephric duct reaches the cloaca by independent caudal growth. (6) The embryonic ureter is an outgrowth of the nephric duct, yet the kidney tubules differentiate from adjacent metanephric blastema.
The kidney and the collecting system originate from the interaction between the mesonephric duct (Wolffian duct) and the metanephric mesenchyme (MM). The uretic bud (UB) forms as an epithelial outpouching from the mesonephric duct and invades the surrounding MM. Reciprocal induction between the UB and MM results in branching and elongation of the UB from the collecting system and in condensation and epithelial differentiation of MM around the branched tips of the UB. Branching of the UB occurs approximately 15 times during human renal development, generating approximately 300,000 and 1 million nephrons per kidney (Nyengaard and Bendtsen, 1992).
This process of reciprocal induction is dependent on the expression of specific factors. Glial cell–derived neurotrophic factor (GDNF) is the primary inducer of ureteric budding (Costantini and Shakya, 2006). GDNF interacts with several different proteins from the MM (eg, Wt-1, Pax2, Eyal, Six1, Sall 1) and from the UB itself (Pax2, Lim1, Ret) resulting in outgrowth of the UB (reviewed by Shah et al, 2004). Proper activation of the Ret/GDNF signaling pathway in the tip of UB epithelium appears to be essential in the progression of branching morphogenesis (reviewed by Michos, 2009). B-catenin and Gata-3 are important regulators of Ret expression, and correct activity of Ret is regulated by positive (Wnt-11 from MM) and negative (Sprouty1 from the UB) feedback signaling. Additional specific factors are required for (1) early branching (eg, Wnt-4 and 11, fgf 7–10); (2) late branching and maturation (bmp2, activin); and (3) branching termination and tubule maintenance (hepatocyte growth factor, transforming growth factor-alpha, epidermal growth factor receptor) (reviewed by Shah et al, 2004). BMP-7, SHH, and Wnt-11 produced from the branching ureteric bud induce the MM to differentiate. These factors induce the activation of Pax2, alpha-8-integrin, and Wnt-4 in the renal mesenchymal cells, resulting in condensation of the MM and the formation of pretubular aggregate and primitive renal vesicle (reviewed by Burrow, 2000). With the continued induction from the UB and the autocrine activity of Wnt-4, the pretubular aggregates differentiate into comma-shaped bodies. Platelet derived growth factor alpha-beta and vascular endothelial growth factor expression are required for initiating the migration of endothelial cells into the cleft of the comma-shaped bodies to form rudimentary glomerular capillary tufts (reviewed by Burrow, 2000). Wt-1 and Pod-1 may have important functions in the regulation of gene transcription necessary for the differentiation of podocytes (Ballermann, 2005).
Anomalies of the Nephric System
Failure of the metanephros to ascend leads to an ectopic kidney. An ectopic kidney may be on the proper side but low (simple ectopy) or on the opposite side (crossed ectopy) with or without fusion. Failure to rotate during ascent causes a malrotated kidney.
Fusion of the paired metanephric masses leads to various anomalies—most commonly a horseshoe kidney.
The ureteral bud from the mesonephric duct may bifurcate, causing a bifid ureter at various levels depending on the time of the bud’s subdivision. An accessory ureteral bud may develop from the mesonephric duct, thereby forming a duplicated ureter, usually meeting the same metanephric mass. Rarely, each bud has a separate metanephric mass, resulting in supernumerary kidneys.
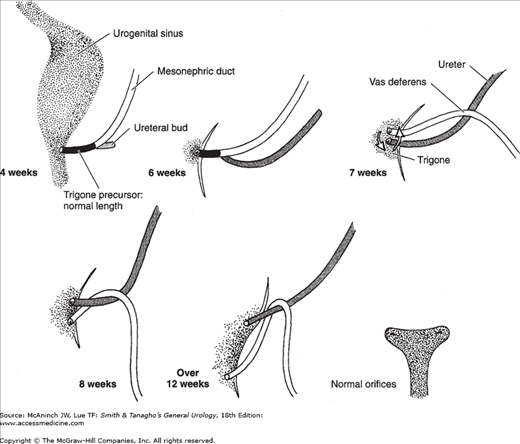
If the double ureteral buds are close together on the mesonephric duct, they open near each other in the bladder. In this case, the main ureteral bud, which is the first to appear and the most caudal on the mesonephric ducts, reaches the bladder first. It then starts to move upward and laterally and is followed later by the second accessory bud as it reaches the urogenital sinus. The main ureteral bud (now more cranial on the urogenital sinus) drains the lower portion of the kidney. The two ureteral buds reverse their relationship as they move from the mesonephric duct to the urogenital sinus. This is why double ureters always cross (Weigert-Meyer law). If the two ureteral buds are widely separated on the mesonephric duct, the accessory bud appears more proximal and ends in the bladder with an ectopic orifice lower than the normal one. This ectopic orifice could still be in the bladder close to its outlet, in the urethra, or even in the genital duct system (Figure 2–3). A single ureteral bud that arises higher than normal on the mesonephric duct can also end in a similar ectopic location.
Figure 2–3.
The development of the ureteral bud from the mesonephric duct and the relationship of both to the urogenital sinus. The ureteral bud appears at the 4th week. The mesonephric duct distal to this ureteral bud is gradually absorbed into the urogenital sinus, resulting in separate endings for the ureter and the mesonephric duct. The mesonephric tissue that is incorporated into the urogenital sinus expands and forms the trigonal tissue.
Vesicourethral Unit
The blind end of the hindgut caudal to the point of origin of the allantois expands to form the cloaca, which is separated from the outside by a thin plate of tissue (the cloacal membrane) lying in an ectodermal depression (the proctodeum) under the root of the tail. At the 4-mm stage, starting at the cephalic portion of the cloaca where the allantois and gut meet, the cloaca progressively divides into two compartments by the caudal growth of a crescentic fold, the urorectal fold. The two limbs of the fold bulge into the lumen of the cloaca from either side, eventually meeting and fusing. The division of the cloaca into a ventral portion (urogenital sinus) and a dorsal portion (rectum) is completed during the 7th week. During the development of the urorectal septum, the cloacal membrane undergoes a reverse rotation, so that the ectodermal surface is no longer directed toward the developing anterior abdominal wall but gradually is turned to face caudally and slightly posteriorly. This change facilitates the subdivision of the cloaca and is brought about mainly by development of the infraumbilical portion of the anterior abdominal wall and regression of the tail. The mesoderm that passes around the cloacal membrane to the caudal attachment of the umbilical cord proliferates and grows, forming a surface elevation, the genital tubercle. Further growth of the infraumbilical part of the abdominal wall progressively separates the umbilical cord from the genital tubercle. The division of the cloaca is completed before the cloacal membrane ruptures, and its two parts therefore have separate openings. The ventral part is the primitive urogenital sinus, which has the shape of an elongated cylinder and is continuous cranially with the allantois; its external opening is the urogenital ostium. The dorsal part is the rectum, and its external opening is the anus.
Traditionally, it is believed that the urogenital sinus receives the mesonephric ducts. The caudal end of the mesonephric duct distal to the ureteral bud (the common excretory duct) is progressively absorbed into the urogenital sinus. By the 7th week, the mesonephric duct and the ureteral bud have independent opening sites. This introduces an island of mesodermal tissue amid the surrounding endoderm of the urogenital sinus. As development progresses, the opening of the mesonephric duct (which will become the ejaculatory duct) migrates downward and medially. The opening of the ureteral bud (which will become the ureteral orifice) migrates upward and laterally. The absorbed mesoderm of the mesonephric duct expands with this migration to occupy the area limited by the final position of these tubes (Figure 2–3). This will later be differentiated as the trigonal structure, which is the only mesodermal inclusion in the endodermal vesicourethral unit.
Recent studies suggest an alternative path of development (reviewed by McInnes and Michaud, 2009). The right and left common excretory duct appears to undergo gradual programmed cell death; the elimination of the common excretory ducts brings the distal ureters in immediate contact with the urogenital sinus epithelium. Concurrently, the ureters undergo a 180° rotation around the axis of the mesonephric duct (also known as the Wolffian duct). The distal segment of the ureters then also undergoes apoptosis. As a result, this process generates a new ureteral connection point in the urogenital sinus region that will give rise to the bladder, while the Wolffian duct remains in the region giving rise to the urethra. Further growth of the bladder and urethra moves the ureteral orifices cranially, while those to the Wolffian ducts move caudally. This pattern of development is supported by recent studies that suggest the trigone is formed mostly from bladder smooth muscle and less so from the ureters. Condensation of myoblasts in the region between the openings of the ureters and Wolffian ducts at 12 weeks of gestation gives rise to the trigone, as a single circular muscular layer and the muscles from the distal ureters cross midline to form the interureteral fold (Oswald et al, 2006).
The urogenital sinus can be divided into two main segments. The dividing line, the junction of the combined Müllerian ducts with the dorsal wall of the urogenital sinus, is an elevation called Müller’s tubercle, which is the most fixed reference point in the whole structure and which is discussed in a subsequent section. The segments are as follows:
The ventral and pelvic portion forms the bladder, part of the urethra in males, and the whole urethra in females. This portion receives the ureter.
The urethral, or phallic, portion receives the mesonephric and the fused Müllerian ducts. This will be part of the urethra in males and forms the lower fifth of the vagina and the vaginal vestibule in females.
During the 3rd month, the ventral part of the urogenital sinus starts to expand and forms an epithelial sac whose apex tapers into an elongated, narrowed urachus. The pelvic portion remains narrow and tubular; it forms the whole urethra in females and the supramontanal portion of the prostatic urethra in males. The splanchnic mesoderm surrounding the ventral and pelvic portion of the urogenital sinus begins to differentiate into interlacing bands of smooth muscle fibers and an outer fibrous connective tissue coat. By the 12th week, the layers characteristic of the adult urethra and bladder are recognizable (Figure 2–4).
Figure 2–4.
Differentiation of the urogenital sinus in males. At the 5th week, the progressively growing urorectal septum separates the urogenital sinus from the rectum. The former receives the mesonephric duct and the ureteral bud. It retains its tubular structure until the 12th week, when the surrounding mesenchyme starts to differentiate into the muscle fibers around the whole structure. The prostate gland develops as multiple epithelial outgrowths just above and below the mesonephric duct. During the 3rd month, the ventral part of the urogenital sinus expands to form the bladder proper; the pelvic part remains narrow and tubular, forming part of the urethra. (Reproduced, with permission, from Tanagho EA, Smith DR: Mechanisms of urinary continence. 1. Embryologic, anatomic, and pathologic considerations. J Urol 1969;100:640.)
The part of the urogenital sinus caudal to the opening of the Müllerian duct forms the vaginal vestibule and contributes to the lower fifth of the vagina in females (Figure 2–5). In males, it forms the inframontanal part of the prostatic urethra and the membranous urethra. The penile urethra is formed by the fusion of the urethral folds on the ventral surface of the genital tubercle. In females, the urethral folds remain separate and form the labia minora. The glandular urethra in males is formed by canalization of the urethral plate. The bladder originally extends up to the umbilicus, where it is connected to the allantois that extends into the umbilical cord. The allantois usually is obliterated at the level of the umbilicus by the 15th week. The bladder then starts to descend by the 18th week. As it descends, its apex becomes stretched and narrowed, and it pulls on the already obliterated allantois, now called the urachus. By the 20th week, the bladder is well separated from the umbilicus, and the stretched urachus becomes the middle umbilical ligament.
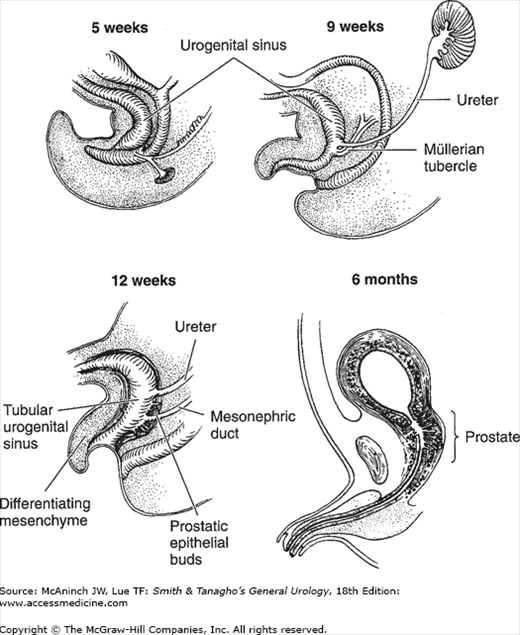
Figure 2–5.
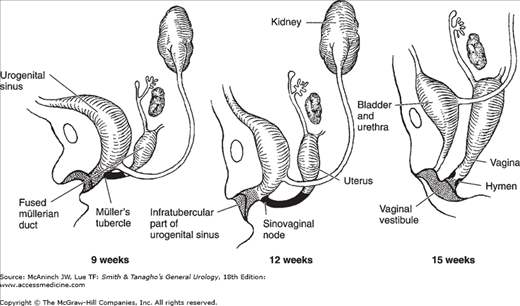
Differentiation of the urogenital sinus and the Müllerian ducts in the female embryo. At 9 weeks, the urogenital sinus receives the fused Müllerian ducts at Müller’s tubercle (sinovaginal node), which is solidly packed with cells. As the urogenital sinus distal to Müller’s tubercle becomes wider and shallower (15 weeks), the urethra and fused Müllerian duct will have separate openings. The distal part of the urogenital sinus forms the vaginal vestibule and the lower fifth of the vagina (shaded area), and that part above Müller’s tubercle forms the urinary bladder and the entire female urethra. The fused Müllerian ducts form the uterus and the upper four-fifths of the vagina. The hymen is formed at the junction of the sinovaginal node and the urogenital sinus.
Prostate
The prostate develops as multiple solid outgrowths of the urethral epithelium both above and below the entrance of the mesonephric duct. These simple tubular outgrowths begin to develop in five distinct groups at the end of the 11th week and are complete by the 16th week (112-mm stage). They branch and rebranch, ending in a complex duct system that encounters the differentiating mesenchymal cells around this segment of the urogenital sinus. These mesenchymal cells start to develop around the tubules by the 16th week and become denser at the periphery to form the prostatic capsule. By the 22nd week, the muscular stroma is considerably developed, and it continues to increase progressively until birth.
From the five groups of epithelial buds, five lobes are eventually formed: anterior, posterior, median, and two lateral lobes. Initially, these lobes are widely separated, but later they meet, with no definite septa dividing them. Tubules of each lobe do not intermingle with each other but simply lie side by side.
The anterior lobe tubules begin to develop simultaneously with those of the other lobes. Although in the early stages, the anterior lobe tubules are large and show multiple branches; gradually, they contract and lose most of the branches. They continue to shrink so that at birth, they show no lumen and appear as small, solid embryonic epithelial outgrowths. In contrast, the tubules of the posterior lobe are fewer in number yet larger, with extensive branching. These tubules, as they grow, extend posterior to the developing median and lateral lobes and form the posterior aspect of the gland, which may be felt rectally.
Prostate development results from complex interaction between urogenital sinus epithelium and mesenchyme in the presence of androgens (reviewed by Cunha et al, 2004, and Thomson, 2008). Early in development, the androgen receptors are solely expressed in the urogenital sinus mesenchyme. Under the influence of androgen, the mesenchyme induces epithelial bud formation, regulates the growth and branching of epithelial bud, promotes differentiation of a secretory epithelium, and specifies differential expression of prostatic secretory proteins. Several members of the fibroblast growth factor (eg, FGF7 and FGF10) play essential role in prostate development, though they do not appear to be directly regulated by androgens. Other factors involved in prostatic duct branching and patterning include Hoxa10, Fut1, and Shh/Ptc. In contrast, other factors such as Activin A serve to inhibit ductal branching in order to maintain tissue homeostasis and regulated growth in the prostate.
Anomalies of the Vesicourethral Unit
Failure of the cloaca to subdivide is rare and results in a persistent cloaca. Incomplete subdivision is more frequent, ending with rectovesical, rectourethral, or rectovestibular fistulas (usually with imperforate anus or anal atresia).
Failure of descent or incomplete descent of the bladder leads to a urinary umbilical fistula (urachal fistula), urachal cyst, or urachal diverticulum depending on the stage and degree of maldescent.
Development of the genital primordia in an area more caudal than normal can result in formation of the corpora cavernosa just caudal to the urogenital sinus outlet, with the urethral groove on its dorsal surface. This defect results in complete or incomplete epispadias depending on its degree. A more extensive defect results in vesical exstrophy. Failure of fusion of urethral folds leads to various grades of hypospadias. This defect, because of its mechanism, never extends proximal to the bulbous urethra. This is in contrast to epispadias, which usually involves the entire urethra up to the internal meatus.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree