Benign Tumors
With the liberal use of computed tomography (CT) scans and magnetic resonance imaging (MRI), benign renal masses are being detected more frequently. Benign renal tumors include, oncocytoma, angiomyolipoma, leiomyoma, lipoma, hemangioma, and juxtaglomerular tumors.
Renal oncocytoma has a spectrum of behavior ranging from benign to malignant. Composed of large epithelial cells with finely granular eosinophilic cytoplasm (oncocytes), oncocytomas occur in various organs and organ systems including adrenal, salivary, thyroid, and parathyroid glands as well as the kidney. An estimated 3–5% of renal tumors are oncocytomas (Romis et al, 2004). Men are affected more often than women.
Renal oncocytomas generally occur and are contained within a well-defined fibrous capsule. Metastasis is extremely rare though invasion of the lymphovascular spaces has been observed. On cut section, the surface of the tumor is usually tan or light brown with a central stellate scar, but necrosis typical of renal adenocarcinoma is absent. The tumors are usually solitary and unilateral, although several bilateral cases and multiple oncocytomas occurring simultaneously (oncocytomatosis) have been reported (Tickoo et al, 1999).
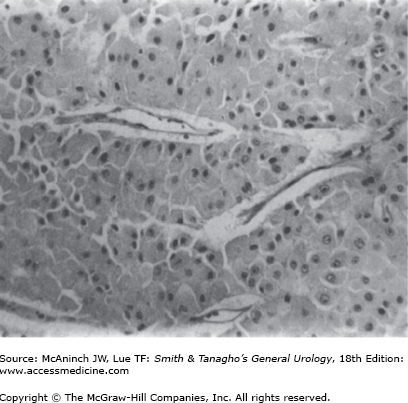
Oncocytomas can also be associated with benign tumors of hair follicles (fibrofolliculomas), colon polyps/tumors, and pulmonary cysts as part of the Birt–Hogg–Dubé syndrome (Toro et al, 1999). The familial renal oncocytoma syndrome has also been described (Philips et al, 2001). These patients may have a characteristic genetic abnormality involving a gene located on 17p encoding a protein named folliculin (Nickerson et al, 2002) Histologically, well-differentiated oncocytomas are made up of large, uniform cells containing an intensely eosinophilic cytoplasm, which on ultrastructural studies is found to be packed with mitochondria. Mitotic activity is absent, and nuclear pleomorphism is uncommon (Figure 22–1). Consistent chromosomal alterations such as loss of chromosome 1 or Y and translocations in the short arm of chromosome 11 have been described in oncocytomas (Lindgren et al, 2004; Philips et al, 2001). The cellular origin of renal oncocytes has not been fully elucidated, although some early evidence suggested that oncocytes resemble proximal convoluted tubular cells (Merino and Librelsi, 1982). Other findings suggest that their origin may be a precursor stem cell (Cohen et al, 1988) or the intercalated cells of the collecting ducts (Storkel et al, 1989).
The diagnosis of oncocytoma is predominantly pathologic because there are no reliable distinguishing clinical characteristics. Gross hematuria and flank pain occur in <20% of patients. No characteristic features of the tumors appear on CT, ultrasound (US), intravenous urography (IVU), or MRI. Angiographic features of oncocytomas include the “spoke-wheel” appearance of tumor arterioles, the “lucent rim sign” of the capsule, and a homogeneous capillary nephrogram phase. Unfortunately, these findings are not invariable, and similar findings have been reported in patients with renal cell carcinoma (RCC).
High-grade oncocytomas may be intermixed with elements of RCC and can be found as coexisting lesions within the same or opposite kidney (Licht et al, 1993). The role of fine-needle aspiration in the preoperative diagnosis of oncocytomas remains controversial and limited due to a lack of characteristic features that distinguish oncocytoma from RCC.
Angiomyolipoma is a rare benign tumor of the kidney seen in two distinct clinical populations. Angiomyolipomas are found in approximately 45–80% of patients with tuberous sclerosis and are typically bilateral and asymptomatic. Tuberous sclerosis is a familial inherited disorder comprising adenoma sebaceum, mental retardation, and epilepsy. In patients without tuberous sclerosis, renal angiomyolipomas can be unilateral and tend to be larger than those associated with tuberous sclerosis (Anderson and Hatcher, 1990). There is no known histologic difference between the lesions seen in these two populations. As many as 25% of cases can present with spontaneous rupture and subsequent hemorrhage into the retroperitoneum (Wong et al, 1981).
Angiomyolipomas are unencapsulated yellow to gray lesions, typically round to oval, that elevate the renal capsule, producing a bulging smooth or irregular mass. They are characterized by three major histologic components: mature fat cells, smooth muscle, and blood vessels. Renal hamartomas may extend to perirenal or renal sinus fat and involve regional lymphatics and other visceral organs (Ditonno et al, 1992). The presence of renal hamartomas in extrarenal sites is a manifestation of multicentricity rather than metastatic potential, because only one well-documented case of malignant transformation of angiomyolipoma has been reported (Lowe et al, 1992).
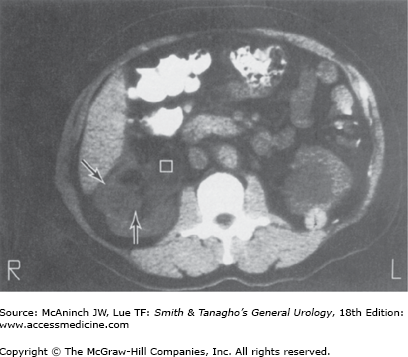
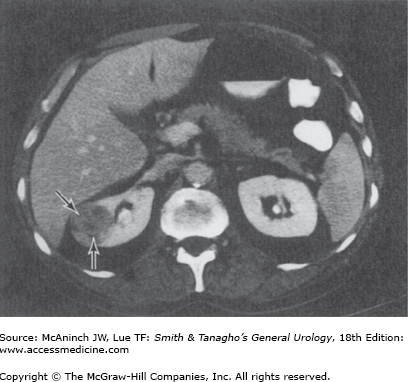
Female patients with a rare condition termed lymphangioleiomyomatosis may have multiple renal and hepatic angiomyolipomata, multiple pulmonary cysts, enlarged abdominal lymph nodes, and lymphangiomyomas (Avila et al, 2000; Urban et al, 1999). The diagnosis of renal angiomyolipoma has evolved with the widespread use of US and CT. Ultrasonography and CT are frequently diagnostic in lesions with high fat content. Fat visualized on US appears as very high intensity echoes. Fat imaged by CT has a negative density, −20 to −80 Hounsfield units, which is pathognomonic for angiomyolipomas when observed in the kidney (Figure 22–2) (Pitts et al, 1980). MRI can also be utilized to identify fat within the lesion and confirm a diagnosis of angiomyolipoma. MRI is particularly useful in distinguishing lipid poor angiomyolipoma which have much lower fat content compared to routine angiomyolipomas, from other solid renal lesions (Kim et al, 2006).
The management of angiomyolipomas historically has been correlated with symptoms. Steiner and colleagues (1993) reported a long-term follow-up study of 35 patients with angiomyolipomas. They proposed that patients with isolated lesions <4 cm be followed up with yearly CT or US. Patients with asymptomatic or mildly symptomatic lesions >4 cm should be followed up with semiannual US. Patients with lesions >4 cm with moderate or severe symptoms (bleeding or pain) should undergo renal-sparing surgery or renal arterial embolization. Recent data indicate that even larger angiomyolipoma’s (AML’s) upto 8 cm can be observed and treated as dictated by symptoms. Approximately 25–30% of patients who are observed will ultimately require treatment in the form of embolization, surgery or radio frequency ablation (Sooriakumaran, 2010). However the long-term efficacy of selective embolization remains suboptimal and the long-term efficacy of radiofrequency ablation is yet to be determined. More recent data also suggests that immunosuppressive agents such as sirolimus (an inhibitor of mammalian target of rapamycin or mTOR) may also be effective in treating AML arising in patients with tuberous sclerosis (Bissler et al, 2008).
Several other benign renal tumors are quite rare, including leiomyomas, hemangiomas, lipomas, and juxtaglomerular cell tumors. With the exception of juxtaglomerular tumors, there are no features that unequivocally establish the diagnosis before surgery; therefore, the pathologist most often provides the diagnosis after nephrectomy.
Leiomyomas are rare small tumors typically found in smooth-muscle-containing areas of the kidney including the renal capsule and renal pelvis. Two groups of renal leiomyomas have been described (Steiner et al, 1990). The more common group comprises cortical tumors that are <2 cm and may be multiple. These tumors are typically found at autopsy and are not clinically significant. A larger, commonly solitary leiomyoma has been described, which may cause symptoms and is confirmed pathologically after nephrectomy.
Hemangiomas are small vascular tumors occurring in the kidney with a frequency second only to that in the liver among visceral organs. Multiple lesions in one kidney occur in approximately 12% of cases; however, they are rarely bilateral. They can occasionally be the elusive source of hematuria in an otherwise well-evaluated patient. The diagnosis may be determined by CT angiography, MR angiography, or by direct visualization by endoscopy (Ekelund and Gothlin, 1975).
Renal lipomas are very uncommon deposits of mature adipose cells without evident mitosis that arise from the renal capsule or perirenal tissue. They are seen primarily in middle-aged females and, owing to the characteristic CT differentiation of fat, are best detected radiographically on CT scanning.
The juxtaglomerular cell tumor is the most clinically significant member of this subgroup of rare benign tumors because it causes significant hypertension that can be cured by surgical treatment. It is a very rare lesion, with <100 reported cases and may have characteristic chromosomal alterations (Brandal et al, 2005). The tumors occur more commonly in young adults, more often females in their 20s and 30s and are rarely malignant. The tumors originate from the pericytes of afferent arterioles in the juxtaglomerular apparatus and can be shown to contain renin secretory granules. They are typically encapsulated and located in the cortical area. Symptoms of the “typical” tumors include hypertension, hypokalemia, hyperaldosteronism, and high renin (Dong et al, 2010). Some atypical cases may demonstrate just hypertension with normal potassium levels or may even be non functional. The diagnosis is confirmed by selected renal vein sampling for renin. Although complete nephrectomy was advocated in the past, several recent reports indicate that partial nephrectomy can be equally effective (Haab et al, 1995).
Adenocarcinoma of the Kidney (RCC)
In the United States in 2010, an estimated 58,240 new cases of adenocarcinoma of the kidney were expected to be diagnosed, and 13,040 deaths were expected to occur from this disease (Jemal et al, 2010). RCC accounts for roughly 2.8% of adult cancers and constitutes approximately 85% of all primary malignant renal tumors. There appears to be an increase in the incidence of all stages of RCC over the past few decades (Hock et al, 2002; Mindrup et al, 2005). RCC occurs most commonly in the fifth to sixth decade and has a male–female ratio of 2:1. The incidence of renal cancer may vary based on race, with black men demonstrating a higher incidence than in men of all other races. Black men may also have a higher likelihood of a subsequent RCC in the contralateral kidney (Rabbani et al, 2002). Asians appear to have the lowest incidence of RCC (Miller, 1996).
The cause of renal adenocarcinoma is unknown. Occupational exposures, chromosomal aberrations, and tumor suppressor genes have been implicated. Cigarette smoking is the only risk factor consistently linked to RCC by both epidemiologic case-control and cohort studies (La Vecchia et al, 1990), with most investigations demonstrating at least a twofold increase in risk for the development of RCC in smokers (Yu et al, 1986). Exposure to asbestos, solvents, and cadmium has also been associated with an increased incidence of RCC (Mandel et al, 1995).
RCC occurs in two forms, inherited and sporadic. In 1979, Cohen and colleagues described a pedigree with hereditary RCC in which the pattern of inheritance was consistent with an autosomal dominant gene with a balanced reciprocal translocation between the short arm of chromosome 3 and the long arm of chromosome 8. Subsequent work has documented that both the hereditary and sporadic forms of RCC are associated with structural changes in chromosome 3p (Erlandsson, 1998; Kovacs et al, 1988; Noordzij and Mickisch, 2004).
Other hereditary forms of RCC have been described. Von Hippel-Lindau disease is a familial cancer syndrome in which affected individuals have a mutation of chromosome 3p predisposition to develop tumors in multiple organs, including cerebellar hemangioblastoma, retinal angiomata, and bilateral clear cell RCC. In 1993, Latif and colleagues identified the von Hippel–Lindau gene, leading to the detection of a germ line mutation in approximately 75% of families affected by von Hippel–Lindau disease (Chen et al, 1995). It has also been recognized that at least 50% of cases of sporadic clear cell RCC has mutations in the Von Hippel–Lindau (VHL) gene (Gnarra et al, 1994).
Hereditary papillary renal carcinoma was described in 1994 and is characterized by a predisposition to develop multiple bilateral renal tumors with a papillary histologic appearance (Zbar et al, 1994). In contrast to von Hippel–Lindau patients, the major neoplastic manifestations appear to be confined to the kidney.
Acquired cystic disease of the kidneys is a well-recognized entity of multiple bilateral cysts in the native kidneys of uremic patients (Reichard et al, 1998). The risk of developing RCC has been estimated to be >30 times higher in patients receiving dialysis who have cystic changes in their kidney than in the general population (Brennan et al, 1991). Several series reported in the literature suggest that RCC occurs in 3–9% of patients with acquired cystic disease of the kidneys (Gulanikar et al, 1998). Most RCC cases have been described in patients undergoing hemodialysis, but RCC has been reported in association with peritoneal dialysis (Smith et al, 1987) and successful renal transplants (Vaziri et al, 1984) and in patients with long-term renal insufficiency not requiring dialysis (Bretan et al, 1986; Fallon and Williams, 1989).
RCC originates from the proximal renal tubular epithelium, as evidenced by electron microscopy (Makay et al, 1987) and immunohistochemical analysis (Holthöfer, 1990). These tumors occur with equal frequency in either kidney and are randomly distributed in the upper and lower poles. RCCs originate in the cortex and tend to grow out into perinephric tissue, causing the characteristic bulge or mass effect that aids in their detection by diagnostic imaging studies. Grossly, the tumor is characteristically yellow to orange because of the abundance of lipids, particularly in the clear cell type. RCCs do not have true capsules but may have a pseudocapsule of compressed renal parenchyma, fibrous tissue, and inflammatory cells.
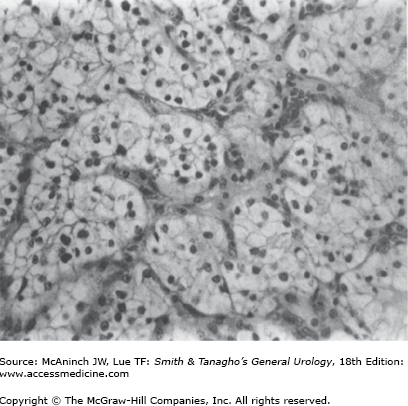
Histologically, RCC is most often a mixed adenocarcinoma containing clear cells, granular cells, and, occasionally, sarcomatoid-appearing cells. The classifications of the subtypes of RCC are based on morphology and cytogenetic characteristics. Most RCCs are classified into one of the following histologic subtypes: conventional clear cell, papillary (chromophilic), chromophobe, collecting duct, neuroendocrine, and unclassified (Mostofi and Davis, 1998). Benign renal tumors are papillary adenoma, renal oncocytoma, and metanephric adenoma. Clear cells are rounded or polygonal with abundant cytoplasm, which contains cholesterol, triglycerides, glycogen, and lipids (Figure 22–3).
The cells present in the papillary (chromophilic) type contain less glycogen and lipids, and electron microscopy reveals that the granular cytoplasm contains many mitochondria and cytosomes. Chromophobe-type carcinomas contain large polygonal cells with distinct cell borders and reticulated cytoplasm, which can stain diffusely with Hale’s colloidal iron (Theones et al, 1988). Oncocytic RCC or oncocytomas tend to have cytoplasm packed with mitochondria, giving it a granular appearance. Collecting duct tumors tend to have irregular borders and a basophilic cytoplasm with extensive anaplasia and are likely to invade blood vessels and cause infarction of tissue. Sarcomatoid cells are spindle shaped and form sheets or bundles. This later cell type rarely occurs as a pure form and is most commonly a small component of either the clear cell or papillary cell type (or both).
RCCs are vascular tumors that tend to spread either by direct invasion through the renal capsule into perinephric fat and adjacent visceral structures or by direct extension into the renal vein. Approximately 25–30% of patients have evidence of metastatic disease at presentation. The most common site of distant metastases is the lung. However, liver, bone (osteolytic), ipsilateral adjacent lymph nodes and adrenal gland, brain, the opposite kidney, and subcutaneous tissue are frequent sites of disease spread.
The ultimate goal of staging is to select appropriate therapy and obtain prognostic information. Appropriate studies for a complete clinical staging evaluation include history and physical examination, complete blood count, serum chemistries (renal and hepatic function), urinalysis, chest x-ray (chest CT scan for an equivocal exam), CT scan of abdomen and pelvis, and a radionuclide bone scan (with x-rays of abnormal areas).
The original staging system described by Robson (1963) is easy to use, but it does not relate directly to prognosis and hence it is no longer commonly used. The tumor-node-metastasis (TNM) system more accurately classifies the extent of tumor involvement and is currently most often used. The TNM classification system for RCC has undergone multiple revisions with the most recent edition being the 2010 version (Table 22–1). In the most recent American Joint Committee on Cancer TNM staging, stage T1 disease is further divided into T1a (tumor size <4 cm) and T1b (size 4–7 cm) as there is a difference in long-term survival between stage T1a and T1b (Ficarra et al, 2005).
Classification | Definition |
|---|---|
T—Primary tumor | |
TX T0 T1 T1a T1b T2 T2a T2b T3 T3a T3b T3c T4 | Primary tumor cannot be assessed No evidence of primary tumor Tumor 7.0 cm or less in greatest dimension, limited to the kidney Tumor less than 4.0 cm in greatest dimension, limited to the kidney Tumor 4.0–7.0 cm in greatest dimension, limited to the kidney Tumor more than 7.0 cm in greatest dimension, limited to the kidney Tumor >7 cm but ≤10 cm in greatest dimension, limited to kidney Tumor >10 cm in greatest dimension, limited to kidney Tumor extends into major veins or perinephric tissues but not into ipsilateral adrenal gland or beyond Gerota’s fascia Tumor invades renal vein or its segmental branches or perirenal fat or renal sinus fat but not beyond Gerota’s fascia Tumor grossly extends into vena cava below the diaphragm Tumor grossly extends into vena cava above diaphragm or into the wall of the vena cava Tumor invades beyond Gerota’s fascia including contiguous extension into ipsilateral adrenal gland |
N—Regional lymph nodes | |
NX N0 N1 | Regional lymph nodes cannot be assessed No regional lymph node metastasis Metastasis in regional lymph nodes |
M—Distant metastases | |
MX M0 M1 | Distant metastasis cannot be assessed No distant metastasis Distant metastasis |
Fuhrman grading has become commonly used by pathologists in North America (Fuhrman et al, 1982; Goldstein, 1997). The system uses four grades based on nuclear size and irregularity and nucleolar prominence. The system is most effective in predicting metastasis (50% of high-grade tumors within 5 years). When high-grade, predominantly granular tumors are corrected for grade and stage, there is no apparent difference between clear cell and granular cell tumor prognosis (McNichols et al, 1981). However, patients presenting with advanced disease do poorly irrespective of tumor grade.
The classically described triad of gross hematuria, flank pain, and a palpable mass occurs in only 7–10% of patients and is frequently a manifestation of advanced disease. Patients may also present with hematuria, dyspnea, cough, and bone pain that are typically symptoms secondary to metastases. With the routine use of CT scanning for evaluation of nonspecific findings, asymptomatic renal tumors are increasingly detected incidentally (>50%).
RCC is associated with a wide spectrum of paraneoplastic syndromes including erythrocytosis, hypercalcemia, hypertension, and nonmetastatic hepatic dysfunction. Overall, these manifestations can occur in 10–40% of patients with RCC.
RCC is the most common cause of paraneoplastic erythrocytosis, which is reported to occur in 3–10% of patients with this tumor (Sufrin et al, 1989). In patients with RCC, the elevated erythrocyte mass is physiologically inappropriate and may result either from enhanced production of erythropoietin from the tumor or as a consequence of regional renal hypoxia promoting erythropoietin production from nonneoplastic renal tissue (Hocking, 1987).
Hypercalcemia has been reported to occur in up to 20% of patients with RCC (Muggia, 1990). Hypercalcemia may be due to production of a parathyroid hormone-related peptide that mimics the function of parathyroid hormone (Strewler et al, 1987) or other humoral factors such as osteoclast-activating factor, tumor necrosis factor, and transforming growth factor-alpha (Muggia, 1990).
Hypertension associated with RCC has been reported in up to 40% of patients (Sufrin et al, 1989), and renin production by the neoplasm has been documented in 37%. The excess renin and hypertension associated with RCC are typically refractory to antihypertensive therapy but may respond after nephrectomy (Gold et al, 1996).
In 1961, Stauffer described a reversible syndrome of hepatic dysfunction in the absence of hepatic metastases associated with RCC. Hepatic function abnormalities include elevation of alkaline phosphatase and bilirubin, hypoalbuminemia, prolonged prothrombin time, and hypergammaglobulinemia. Stauffer’s syndrome tends to occur in association with fever, fatigue, and weight loss and typically resolves after nephrectomy. The reported incidence of Stauffer’s syndrome varies from 3% to 20% (Gold et al, 1996). It may be due to overproduction of granulocyte-macrophage colony stimulating factor by the tumor (Chang et al, 1992).
RCC is known to produce a multitude of other biologically active products that result in clinically significant syndromes, including adrenocorticotropic hormone (Cushing’s syndrome), enteroglucagon (protein enteropathy), prolactin (galactorrhea), insulin (hypoglycemia), and gonadotropins (gynecomastia and decreased libido; or hirsutism, amenorrhea, and male pattern balding) (Sufrin et al, 1986).
A paraneoplastic syndrome present at the time of disease diagnosis does not, in and of itself, confer a poor prognosis. However, patients whose paraneoplastic metabolic disturbances fail to normalize after nephrectomy (suggesting the presence of clinically undetectable metastatic disease) have very poor prognoses (Hanash, 1982).
In addition to the laboratory abnormalities associated with the various RCC paraneoplastic syndromes, anemia, hematuria, and an elevated sedimentation rate are frequently observed.
Anemia occurs in about 30% of RCC patients. The anemia typically is not secondary to blood loss or hemolysis and is commonly normochromic. The serum iron and total iron-binding capacity are usually low, as in the anemia of chronic disease. Iron therapy is usually ineffective; however, surgical removal of early-stage tumors usually leads to physiologic correction of the anemia. The potential role of recombinant erythropoietin for patients with unresectable disease represents a potential, but untested, option.
Gross or microscopic hematuria can be seen in up to 60% of patients presenting with RCC. An elevated erythrocyte sedimentation rate is also commonly seen, with a reported incidence as high as 75%. These findings are nonspecific, and normal findings do not rule out a diagnosis of RCC.
Although many radiologic techniques are available to aid in the detection and diagnosis of renal masses, CT scanning remains the primary technique with which others must be compared. Other radiologic techniques used include US and MRI.
US examination is a noninvasive, relatively inexpensive technique able to further delineate a renal mass. It is approximately 98% accurate in distinguishing simple cysts from solid lesions. Strict ultrasonographic criteria for a simple cyst include through transmission, a well-circumscribed mass without internal echoes, and adequate visualization of a strong posterior wall (Figure 22–4).
CT scanning is more sensitive than US for detection of renal masses. A typical finding of RCC on CT is a mass that becomes enhanced with the use of intravenous contrast media. In general, RCC exhibits an overall decreased density in Hounsfield units compared with normal renal parenchyma but shows either a homogeneous or heterogeneous pattern of enhancement (increase in density of >10 Hounsfield units) following contrast administration (Figure 22–5). In addition to defining the primary lesion, CT scanning is also the method of choice in staging the patient by visualizing the renal hilum, perinephric space, renal vein and vena cava, adrenals, regional lymphatics, and adjacent organs. In patients with equivocal chest x-ray findings, a CT scan of the chest is indicated. Patients who present with symptoms consistent with brain metastases should be evaluated with either head CT or MRI. Spiral CT with 3-dimensional reconstruction has become useful for evaluating tumors before nephron-sparing surgery to delineate the 3-dimensional extent of the tumor and precisely outline the vasculature, which can aid the surgeon in preventing positive surgical margins (Holmes et al, 1997). Intraoperative ultrasonography is also often used to confirm the extent and number of masses in the kidney at the time of performing a partial nephrectomy.
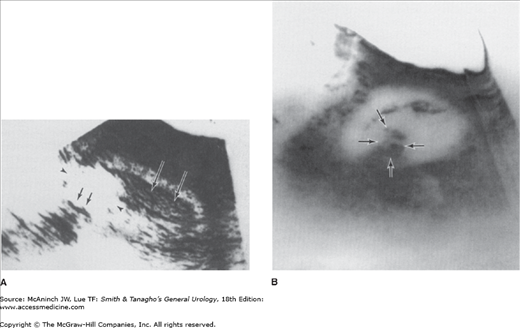
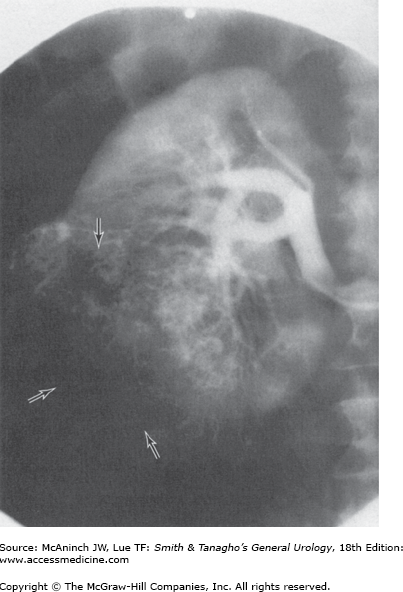
With the widespread availability of CT scanners, the role of renal angiography in the diagnostic evaluation of RCC has markedly diminished and is now very limited. There remain a very few specific clinical situations in which angiography may be useful; for example, guiding the operative approach in a patient with an RCC in a solitary kidney when attempting to perform a partial nephrectomy may be indicated (Figure 22–6). However, CT angiography or MR angiography can give better information with less risk to the patient.
Determination of metastases to bones is most accurate by radionuclide bone scan, although the study is nonspecific and requires confirmation with bone x-rays of identified abnormalities to verify the presence of the typical osteolytic lesions. There is evidence that patients without bone pain and with a normal alkaline phosphatase level have a very low incidence of bone metastases (Henriksson et al, 1992), and thus a routine bone scan is not necessary in such patients.
MRI is equivalent to CT for staging of RCC (Hricak et al, 1988). Its primary advantage is in the evaluation of patients with suspected vascular extension (Figure 22–7). Prospective trials have demonstrated that MRI is superior to CT in assessing inferior vena caval involvement (Kabala et al, 1991) and is at least as accurate as venacavography (Horan et al, 1989). In contrast to both CT and cavography, MRI evaluation does not require either iodinated contrast material or ionizing radiation. Recent studies using MRI angiography with gadolinium or CT angiography have improved vascular evaluation of renal neoplasms (Bluemke and Chambers, 1995). MR angiography can also be used to delineate the vascular supply before planned nephron-sparing surgery.
This technique allows the measurement of systemically administered biochemical agents such as 18-fluoro-2-deoxyglucose (FDG), which can accumulate in the kidney. Although FDG-PET scanning can yield false-positive results in some patients with RCC (Bachor et al, 1996), it may be useful in monitoring response to systemic therapy in those with metastatic disease (Hoh et al, 1998). FDG-PET may also be more accurate than routine CT scanning in detecting disease recurrence or progression, which may alter treatment decisions in up to 50% of cases (Ramdave et al, 2001). However, most recent studies suggest that FDG-PET is of insufficient sensitivity to be useful for staging RCC.
The enzyme carbonic anhydrase IX (CA IX) is expressed at high levels in clear cell RCC. CA IX is regulated by the VHL gene through HIF1α and with loss of the VHL tumor suppressor gene being very common in clear cell RCC, there is loss of regulation of CA IX expression which is significantly increased. It is expressed at low levels in the gastrointestinal, mucosa, and biliary tract but not in other normal tissues. This characteristic can be exploited to detect clear cell RCC using a radiolabeled monoclonal antibody scan using the antibody G250 which targets carbonic anhydrase IX (Stillebroer et al, 2010). Up to 80% of clear cell RCC are found to express G250 antigen and in nearly imaging studies (Oosterwijnk et al, 1993). Other renal tumors such as chromophobe and papillary RCC demonstrate very little CA IX expression. More recently an immuno-PET approach has been used combining the monoclonal antibody to CA IX and a PET scan to better visualize renal lesions. In early studies, 94% of renal tumors were correctly identified using this approach (Divgi et al, 2007).
Fine-needle aspiration of renal lesions is the diagnostic approach of choice in those patients with clinically apparent metastatic disease who may be candidates for nonsurgical therapy. Other settings in which fine-needle aspiration may be appropriate include establishing a diagnosis in patients who are not surgical candidates, differentiating a primary RCC from a renal metastasis in patients with known primary cancers of nonrenal origin, and evaluating some radiographically indeterminate lesions. Fine needle aspiration is being increasingly used to confirm the diagnosis of a neoplasm particularly in patients who may undergo observation or percutaneous ablative therapy (Shah et al, 2005). While core needle biopsies may be able to accurately diagnose malignancy in up to 100% of cases >4 cm and 95% of cases <4 cm, this may require multiple cores for accuracy (Wunderlich et al, 2005). Rare reports of seeding of the needle tract have been reported but the risk of seeding is reported to be <0.01% (Volpe et al, 2007). Recently, core biopsies of the primary renal mass have been more commonly utilized in patients with metastatic disease (when biopsying a metastatic site is not feasible), in order to guide appropriate targeted systemic therapy (prior to or instead of cytoreductive nephrectomy), since the choice of systemic therapy can be influenced by the specific RCC histology. The accuracy of needle core biopsies is reported to be >90% with sensitivity ranging between 70–100% and specificity of 100% (Volpe et al, 2007). The accuracy of fine needle aspiration cytology of renal masses is reported to be slightly lower mainly because of lower sensitivity. Specificity can still be high and close to 100%.
Patients presenting with hematuria should also be evaluated with cystoscopy. Blood effluxing from the ureteral orifice identifies the origin of bleeding from the upper tract. Most renal pelvis tumors can be distinguished radiographically from RCC; however, endoscopic evaluation of the bladder, ureters, and renal pelvis is occasionally helpful in making a diagnosis. In addition, although urine cytologic study is rarely helpful in the diagnosis of RCC, cytologic study of urine with renal pelvis washing is frequently diagnostic in renal pelvis tumors.
When a patient presents with clinical findings consistent with metastatic disease and is found to have a renal mass, a diagnosis of RCC can be straightforward. Most patients present with a renal mass discovered after an evaluation of hematuria or pain or as an incidental finding during an imaging workup of an unrelated problem. The differential diagnosis of RCC includes other solid renal lesions. The great majority of renal masses are simple cysts. Once the diagnosis of a cyst is confirmed by US, no additional evaluation is required if the patient is asymptomatic. Equivocal findings or the presence of calcification within the mass warrant further evaluation by CT. A wide variety of pathologic entities appear as solid masses on CT scans, and differentiation of benign from malignant lesions is frequently difficult. Findings on CT scan that suggest malignancy include amputation of a portion of the collecting system, presence of calcification, a poorly defined interface between the renal parenchyma and the lesion, invasion into perinephric fat or adjacent structures, and the presence of abnormal periaortic adenopathy or distant metastatic disease. The frequency of benign lesions among renal masses <7 cm in size is as high as 16–20% (Duchene et al, 2003; Snyder et al, 2006). Masses >7 cm are rarely benign.
Some characteristic lesions can be defined using CT criteria in combination with clinical findings. Angiomyolipomas (with large fat components) can easily be identified by the low-attenuation areas classically produced by substantial fat content. A renal abscess may be strongly suspected in a patient presenting with fever, flank pain, pyuria, and leukocytosis, and an early needle aspiration and culture should be performed. Other benign renal masses (in addition to those previously described) include granulomas and arteriovenous malformations. Renal lymphoma (both Hodgkin’s disease and non-Hodgkin’s disease), transitional cell carcinoma of the renal pelvis, adrenal cancer, and metastatic disease (most commonly from a lung or breast cancer primary) are additional diagnostic possibilities that may be suspected based on CT and clinical findings.
Surgical removal of the early-stage lesion remains the only potentially curative therapy available for RCC patients. Appropriate therapy depends almost entirely on the stage of tumor at presentation and therefore requires a thorough staging evaluation. The prognoses of patients with stages T1–T3a disease are similar following radical nephrectomy.
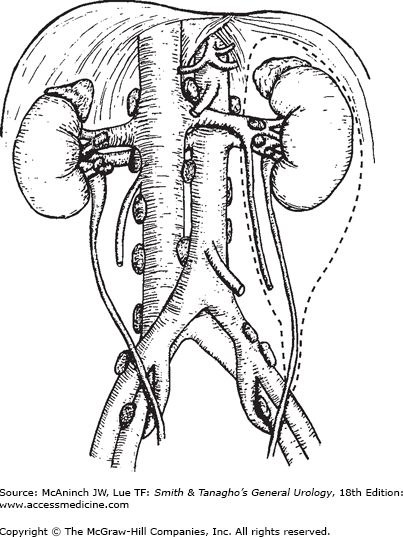
Radical or partial nephrectomies are the primary treatments for localized RCC. The goal is to achieve the removal of tumor and to take a wide margin of normal tissue. Radical nephrectomy entails en bloc removal of the kidney and its enveloping fascia (Gerota’s) including the ipsilateral adrenal, proximal one-half of the ureter, and lymph nodes up to the area of transection of the renal vessels (Figure 22–8).
Various open incisions provide optimal access for the radical nephrectomy, including an anterior subcostal (unilateral chevron) or thoracoabdominal incision, and, occasionally, a midline incision or the classic flank incision.
The likelihood of local recurrence after radical nephrectomy is 2–3% (Itano et al, 2000). Repeat resection of isolated local recurrence can be curative and yield a survival benefit (Itano et al, 2000; Tanguay et al, 1996). The role of regional lymphadenectomy in RCC remains controversial. Between 18% and 33% of patients undergoing radical nephrectomy with lymph node dissection for RCC have metastatic disease identified (Skinner et al, 1988). Although several retrospective studies (Thrasher and Paulson, 1993) and a prospective, nonrandomized study (Herrlinger et al, 1991) suggest that regional lymphadenectomy can improve survival in patients with T1–T2 RCC. More recent studies including a randomized prospective study as well as a population-based study failed to show any survival benefit that could be obtained by routinely performing regional lymphadenectomy especially in patients with organ-confined disease (Blom et al, 1999; Joslyn et al, 2005). Removal of the adrenal is unnecessary if the tumor is not in the upper pole, because adrenal involvement is uncommon in this instance.
Preoperative renal artery embolization (angioinfarction) has been used in the past as a surgical adjunct to facilitate radical nephrectomy, but because there is no conclusive evidence that preoperative embolization actually decreases blood loss or facilitates surgery, its use should be limited to patients with very large tumors in which the renal artery may be difficult to reach early in the procedure. In addition, this technique may be useful to palliate patients with nonresectable tumors and significant symptoms such as hemorrhage, flank pain, or paraneoplastic syndromes.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree