Introduction
The proportion of individuals older than 65 years will rise significantly over the next 25 years. According to US census data, the number of Americans older than 65 years will rise from approximately 35 million today (12.4% of all Americans) to nearly 55 million in 2020 (16.3% of total), and nearly 87 million in 2050 (20.7% of total). As a consequence, the health care system will likely experience a dramatic increase in age-related health problems, such as cancer, cerebrovascular and ischemic heart disease, and hormone deficiency. A substantial body of literature supports the assertion that hormonal changes in the aging male can be associated with significant health problems. This chapter reviews the epidemiology of testosterone deficiency in older men; alterations in testis biology that occur with age; and the effects that these changes may have on semen quality, fertility, birth defects in offspring, and on the overall health of older men.
Epidemiology
Hypogonadism may affect up to 4 million Americans, with a minority receiving treatment. From a population standpoint, the effect of aging on circulating testosterone levels is highly significant. The Baltimore Longitudinal Study of Aging (BLSA) found that 12%, 20%, 30%, and 50%, respectively, of men in their 50s, 60s, 70s, and 80s were hypogonadal using a total serum testosterone threshold of 325 ng/dL. Age has been shown to be an independent risk factor for hypogonadism even after adjustment for chronic medical conditions, such as obesity, diabetes mellitus, and hyperthyroidism.
Changes in Testis Biology with Age
The quest to understand the underlying mechanism behind the epidemiological and clinical observations that aging men experience a gradual decline in testosterone levels led to the analysis of Leydig cell populations in human testes. Leydig cells produce 95% of adult male testosterone and are found in the interstitial space between seminiferous tubules. Kaler and Neaves (1978), in a study of testes retrieved at autopsy after sudden death among men aged 18–87, noted that total Leydig cell volume declines significantly with age and that decline is directly proportional to a fall in the total number of Leydig cells. From such studies, it is estimated that a pair of young testes (20-year-old) contains 700 million Leydig cells and undergoes an attrition of about 80 million cells per decade of life. Other studies have also shown that serum luteinizing hormone (LH) levels are significantly higher in older men compared with younger men, providing physiologic corroboration of the Leydig cell studies.
Although the decline of testosterone levels in older males has been described by the terms andropause, male climacteric, male menopause, late onset hypogonadism (LOH), and partial androgen deficiency in the aging male (PADAM), only LOH and PADAM accurately reflect these changes. Serum testosterone levels in men fall progressively from the third decade to the end of life, primarily due to the decline in testicular Leydig cell mass. This decline may be associated with changes in the circadian rhythm and hypothalamic–pituitary homeostatic control of LH secretion, which regulates testosterone production. Thus, mechanisms exist both at the testicular and hypothalamic–pituitary levels that may result in decreased testosterone with age.
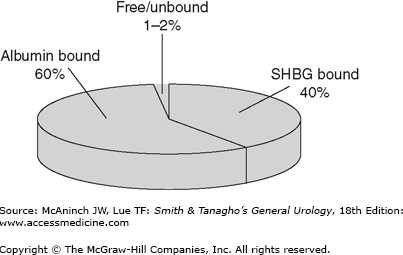
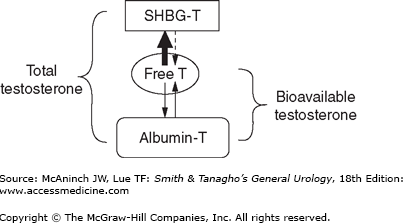
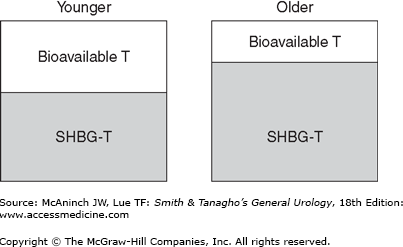
Testosterone exists in several different forms in plasma, with each having different bioactivity (Figure 45–1). Free or unbound testosterone is fully bioavailable, but protein-bound testosterone is only partly bioavailable. Among protein-bound forms, albumin-bound testosterone is more freely bioavailable than sex hormone-binding globulin (SHBG)-bound testosterone, which is considered an inactive form of testosterone (Figure 45–2). Population-based studies have demonstrated that aging and several medical comorbidities are associated with an increase in SHBG. This increase in SHBG leads to increased binding to and inactivation of testosterone, lowering the levels of bioavailable androgens (Figure 45–3). Fifty percent of men older than 60 years have below normal levels of non–SHBG-bound testosterone. The age-related onset, rate, and degree of change in testosterone production are all variable; however, a general rule of thumb is that mean testosterone levels decrease approximately 1% annually after the age of 50. Indeed, the concentration of bioavailable testosterone in men decreases by as much as 50% between the ages of 25 and 75 years. In contrast, dihydrotestosterone (DHT) and estradiol levels (a primary metabolite of testosterone and potent androgen) show minimal and mild declines, respectively, as men age.
Figure 45–2.
Diagram outlining the various forms of testosterone in the blood. Total testosterone includes all forms of the hormone, both free and bound. The affinity of sex hormone-binding globulin (SHBG) for testosterone is much higher (thick arrow) than that of albumin. Available forms of testosterone that exert physiologic activity include free and albumin-bound fractions. T, testosterone.
Age-related declines in Sertoli cells have been observed in anatomical studies. These studies revealed that Sertoli cell populations in the young adult male testis have approximately 500 million Sertoli cells compared with 300 million for older men (Table 45–1). Concurrent declines in testicular mass and the numbers of round spermatids have also been observed.
Age range | ||
|---|---|---|
Testis parameter | 20–48 years | 50–85 years |
Average testis weight | 19 g | 16 g |
No. of Sertoli cells/testis | 503 million | 312 million |
No. of round spermatids | 55 million/g testis | 41 million/g testis |
No. of spermatids/Sertoli cell | 4.0 | 4.3 |
Spermatogenesis
The changes that occur in the seminiferous tubules with age include a decrease in total volume of the testis occupied by seminiferous tubules and a decrease in actual tubule length. Calculations of mature sperm production in testis tissue homogenates also suggest that daily sperm production decreases significantly with age (Table 45–2). The age-related decrease in sperm production in older testes appears to result from a decrease in primary spermatocytes or a decrease in spermatogonial proliferation rather than cellular degeneration. Correspondingly, follicle-stimulating hormone (FSH) levels increase significantly with age, with mean values threefold higher in older men than in younger men.
Age range | ||
|---|---|---|
Testis parameter | 20–48 years | 50–90 years |
Paired testis weight | 41 g | 31 g |
Seminiferous tubule volume | 24 mL/person | 18 mL/person |
Daily sperm production | 250 million | 121 million |
Although an age-related decrease in semen quality might be expected from calculated changes in testis biology with age, this has not been obvious to demonstrate clinically. Cross-sectional studies have observed both decreased and unchanged ejaculated sperm concentrations in older versus younger men. However, most studies show that sperm motility is consistently lower in older men compared with younger men, with a decrease of approximately 0.7% motility per year after the third decade. These declines can be difficult to attribute to age, given the wide variability in semen analysis results between repeated samples from the same individual and the lack of prospective, population-based studies to confirm the findings found in cross-sectional analyses.
The effect of paternal age on fertility is controversial. Studies that address this issue are confounded by the variables of the aging female partner and decreased coital frequency that occurs with age. Despite these limitations, several studies have demonstrated delays in the time to achieve a pregnancy in men older than 35 compared with men younger than 30, even after controlling for maternal age. Advancing paternal age has also been implicated as a risk factor for developmental and chromosomal abnormalities in their offspring. The mechanism of these declines is not yet clear; however, researchers have demonstrated that increased levels of reactive oxygen species are found in the semen of older men in addition to higher levels of genetic abnormalities.
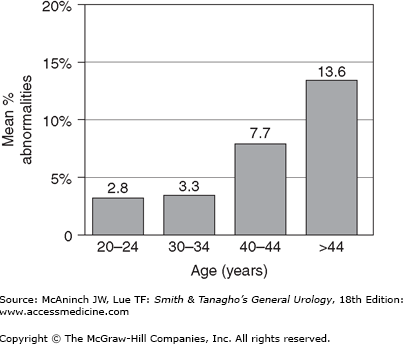
Early sperm cytogenetic studies in fertile men stratified by age showed a 10% overall incidence of sperm chromosomal abnormalities, but no relationship between paternal age and the frequency of numerical abnormalities (aneuploidy) in sperm chromosomes. Recent studies using more sensitive fluorescence in situ hybridization (FISH) technology have shown more subtle paternal age effects on sperm aneuploidy. Interestingly, paternal age appears to increase the fraction of sperm with sex chromosomal aneuploidies. Even more pronounced is the highly significant positive, linear relationship demonstrated between paternal age and the frequency of structural anomalies in sperm (r= 0.63, Figure 45–4).
It is possible that continued cell divisions that characterize spermatogenesis place the germ cells at risk for chromosomal injury, especially given the extended exposure to clastogens, such as reactive oxygen species, that occur with age. However, it is important to realize that studies of live newborns or prenatally diagnosed fetuses do not support the assertion that de novo structural chromosomal anomalies, with the exception of inherited reciprocal translocations, are found more commonly in the offspring of older men.
Single gene defects in sperm can result from errors in the DNA replication process. Numerous studies have cataloged the association between advanced paternal age and new cases of conditions associated with single gene deletions (Table 45–3). One mechanism for the development of new single gene mutations with advanced paternal age implicates the characteristic and continuous process of spermatogonial cell division in spermatogenesis. By puberty, 30 cell divisions of spermatogonia have occurred, resulting in a large pool of undifferentiated cells. After puberty, 23 divisions per year occur; in a 35-year-old man, these cells will have undergone 540 divisions. The simple fact that the spermatogonial stem cells of older men having undergone numerous cell divisions may make them more likely to contain errors in DNA transcription, the source of single gene defects.
Achondroplasias | Aniridia |
Apert syndrome | Bilateral retinoblastoma |
Crouzon syndrome | Fibrodysplasia ossificans |
Hemophilia A | Lesch-Nyhan syndrome |
Marfan syndrome | Neurofibromatosis |
Oculodentodigital syndrome | Polycystic kidney disease |
Polyposis coli | Progeria |
Treacher-Collins syndrome | Tuberous sclerosis |
Waardenburg syndrome |
There is little doubt of the association between advanced paternal age and an increased probability of autosomal-dominant diseases in offspring (Table 45–3). This risk of autosomal mutations due to advanced paternal age has been quantified by investigators who demonstrated that for men aged 40–44, the risk of a mutation occurring in offspring was 4.5 per 1000 births compared with 0.22 per 1000 births among men younger than 29. Analogously, aneuploid conceptions associated with female age demonstrate a similar pattern. It has been suggested that this underlying genetic mechanism may explain the consistent association between paternal age and schizophrenia found among offspring from a range of countries around the world.
Paternal age has been the source of several studies of anatomic birth defects. One demonstrated that paternal age older than 40 was associated with a 20% increase in the incidence of having offspring with a serious birth defect, such as situs inversus, atrial septal defects, or ventricular septal defects. Because of these relationships, guidelines for sperm donors recommend that donors be younger than 50 years.