Urologic Laboratory Examination: Introduction
Examination of specimens of urine, blood, and genitourinary secretions or exudates commonly directs the subsequent urologic workup and frequently establishes a diagnosis. Since approximately 20% of patients who visit a primary physician’s office have a urologic problem, it is important for the physician to have a broad knowledge of the laboratory methods available to test appropriate specimens. Judicious use of such tests permits rapid, accurate, and cost-effective determination of the probable diagnosis and directs the management of patients with urologic disease.
Examination of Urine
Urinalysis is one of the most important and useful urologic tests available, yet all too often, the necessary details are neglected and significant information is overlooked or misinterpreted. Reasons for inadequate urinalyses include (1) improper collection, (2) failure to examine the specimen immediately, (3) incomplete examination (eg, most laboratories do not perform a microscopic analysis unless it is specifically requested by the provider), (4) inexperience of the examiner, and (5) inadequate appreciation of the significance of the findings.
The necessity of routine urinalysis as a screen in asymptomatic individuals, those admitted to hospitals, or those undergoing elective surgery continues to be debated. Numerous studies indicate that in these situations, urinalysis is not routinely necessary (Godbole and Johnstone, 2004). However, patients presenting with urinary tract symptoms or signs should undergo urinalysis. Studies also indicate that, if macroscopic urinalysis (dip-strip) is normal, microscopic analysis is not necessary. If the patient has signs or symptoms suggestive of urologic disease, or the dip-strip is positive for protein, heme, leukocyte esterase, or nitrite, a complete urinalysis, including microscopic examination of the sediment, should be carried out (Simerville et al, 2005).
It is best to examine urine that has been properly obtained in the office. First-voided morning specimens are helpful for qualitative protein testing in patients with possible orthostatic proteinuria and for specific-gravity assessment as a presumptive test of renal function in patients with minimal renal disease due to diabetes mellitus or sickle cell anemia or in those with suspected diabetes insipidus. Evaluation of sequential morning specimens may be required to obviate the variability often encountered. Urine specimens that are obtained immediately after the patient has eaten or that have been left standing for a few hours become alkaline and thus may contain lysed red cells, disintegrated casts, or rapidly multiplying bacteria; therefore, a freshly voided specimen obtained a few hours after the patient has eaten and examined within 1 hour of voiding is most reliable. The patient’s state of hydration may alter the concentration of urinary constituents. Timed urine collections may be required for definitive assessment of renal function or proteinuria.
Proper collection of the specimen is particularly important when patients have hematuria or proteinuria or are being evaluated for urinary tract infection. Examination of a urine specimen collected sequentially during voiding in several containers may help to identify the site of origin of hematuria or urinary tract infection. To gather consistent and meaningful urinalysis data, urine must be collected by a uniform method in the physician’s office or laboratory. The specimen should be obtained before a genital or rectal examination in order to prevent contamination from the introitus or expressed prostatic secretions. Urine obtained from a condom, chronic catheter, or intestinal conduit drainage bag is not a proper specimen for urinalysis.
It is usually simple to collect a clean-voided midstream urine sample from men. Routine instructions may be printed on a sheet given to the patient or placed on the lavatory wall. The procedure should include (1) retraction of the foreskin (a common source of contamination of the specimen) and cleansing of the meatus with benzalkonium chloride or hexachlorophene, (2) passing the first part of the stream (15–30 mL) without collection, and (3) collecting the next or midstream portion (approximately 50–100 mL) in a sterile specimen container, which is capped immediately afterward. A portion of the specimen is prepared immediately for both macroscopic and microscopic examination, and the rest is saved in the sterile container for subsequent culture if this proves necessary.
With this midstream clean-catch method, the likelihood that the specimen will be contaminated by meatal or urethral secretions is markedly decreased, although not completely eliminated. In adult males, it is rarely necessary to collect urine by catheterization unless urinary retention is present.
The best method for collecting a clean-voided midstream specimen from a woman is as follows: (1) the patient is placed on the examining table in the lithotomy position; (2) the vulva and urethral meatus are cleansed with benzalkonium chloride or hexachlorophene; (3) the labia are separated; and (4) the patient is instructed to initiate voiding into a container held close to the vulva. After she has passed the first 10–20 mL of urine, the next 50–100 mL is collected in a sterile container that is immediately capped. Because this technique requires considerable effort, it is acceptable to have the patient provide an initial specimen in a nonsterile container in the office lavatory. If results of urinalysis are normal, no further study is indicated; if abnormal, a urine specimen must be obtained by the more exacting technique. In either case, the specimen should be prepared for immediate examination.
If a satisfactory specimen cannot be obtained by the method described earlier, one should not hesitate to obtain a specimen by catheterization to eliminate nonvaginal sources of abnormal urinary constituents.
Urine for analysis, other than bacterial cultures, can be obtained from males or females by covering the cleansed urethral meatus with a plastic bag; a urine specimen for culture may require catheterization or suprapubic needle aspiration. In girls, catheterization with a small catheter attached to a centrifuge tube is appropriate, but boys should not be routinely catheterized. It is often preferable in either sex to proceed with suprapubic needle aspiration. This is easier if the patient has been previously hydrated, so that the bladder is full. Suprapubic needle aspiration is performed as follows. (1) Cleanse the suprapubic area by sponging with alcohol. (2) With a small amount of local anesthetic, raise an intradermal wheal on the midline 1–2 cm above the pubis (the bladder lies just above the pubis in young children). (3) Attach a 10-mL syringe to a 22-gauge needle. Insert the needle perpendicularly through the abdominal wheal into the bladder wall, maintaining gentle suction with the syringe so that urine will be aspirated as soon as the bladder is entered.
Macroscopic examination of urine often provides a clue when diagnosis is difficult.
Urine is often colored owing to drugs: phenazopyridine (Pyridium) will turn the urine orange; rifampin will turn it yellow-orange; nitrofurantoin will turn it brown; and l-dopa, α-methyldopa, and metronidazole will turn it reddish-brown. Red urine does not always signify hematuria. A red discoloration unassociated with intact erythrocytes in the urine can result from betacyanin excretion after beet ingestion, phenolphthalein in laxatives, ingestion of vegetable dyes, concentrated urate excretion, myoglobinuria due to significant muscle trauma, or hemoglobinuria following hemolysis. In addition, Serratia marcescens bacteria can cause the “red diaper” syndrome. However, whenever red urine is seen, hematuria must be ruled out by microscopic analysis. Cloudy urine is commonly thought to represent pyuria, but more often the cloudiness is due to large amounts of amorphous phosphates, which disappear with the addition of acid, or urates, which dissolve with the use of alkali. The odor of urine is rarely clinically significant.
The specific gravity of urine (normal, 1.003–1.030) is often important for diagnostic purposes: that of patients with significant intracranial trauma may be low owing to a lack of antidiuretic hormone (vasopressin); that of patients with primary diabetes insipidus is <1.010 even after overnight dehydration; that of patients with extensive acute renal tubular damage is consistently 1.010 (similar to the specific gravity of plasma); and a low specific gravity can be an early sign of renal damage from conditions such as sickle cell anemia. Urine specific gravity is the simplest time-honored test for evaluating hydration in postoperative patients. The specific gravity of urine may affect the results of other urine tests: in dilute urine, a pregnancy test may be falsely negative; in concentrated urine, protein may be falsely positive on dip-strips yet unconfirmed on quantitative tests. The specific gravity of urine may be falsely elevated by the presence of glucose, protein, artificial plasma expanders, or intravenous contrast agents.
Studies of specific-gravity reagent strips (method based on ionic alteration of a polyelectrolyte solution) have shown the method to be rapid, reliable, and unaffected by elevated amounts of glucose or contrast medium; however, alkaline pH may falsely lower the result (0.005 per pH unit >7.0). In the routine office setting, these strips are as reliable as either the hydrometer or refractometer methods.
Chemically impregnated reagent strips are accurate and have simplified routine urinalysis greatly. However, they must be monitored routinely by appropriate standardized quality-control reagents. The dip-strips are reliable only when not outdated and when used with room temperature urine.
The pH of urine is important in a few specific clinical situations. Patients with uric acid stones rarely have a urinary pH >6.5 (uric acid is soluble in alkaline urine). Patients with calcium stones, nephrocalcinosis, or both may have renal tubular acidosis and will be unable to acidify urine pH <6.0. With urinary tract infections caused by urea-splitting organisms (most commonly Proteus species), the urinary pH tends to be >7.0. It should be reemphasized that urine obtained within 2 hours of a large meal or left standing at room temperature for several hours tends to be alkaline. The indicator paper in most dip-strips is quite accurate; however, confirmation by a pH meter is occasionally required.
Dip-strips containing bromphenol blue can be used to determine the presence of >10 mg/dL protein in urine, but persistent proteinuria detected in this manner requires quantitative protein testing for confirmation. The dip-strip measures primarily albumin and is not sensitive to Bence-Jones proteins (immunoglobulins). Concentrated urine may give a false-positive result, as will urine containing numerous white blood cells (leukocytes) or vaginal secretions replete with epithelial cells. Orthostatic proteinuria can be demonstrated by detecting elevated protein levels in a urine specimen obtained after the patient has been in the upright position for several hours, whereas normal levels are found before ambulation. Prolonged fever and excessive physical exertion are also common causes of transient proteinuria.
Persistently elevated protein levels in the urine (>150 mg/24 h) may indicate significant disease. Therefore, specific quantitative protein tests, electrophoretic studies of the urine, or both may be required to determine the specific type of protein that is present.
The glucose oxidase–peroxidase tests used in dip-strips are quite accurate and specific for urinary glucose. False-positive results may be obtained when patients have ingested large doses of aspirin, ascorbic acid, or cephalosporins. An occasional patient has a blood glucose level below 180 mg/dL and yet has significant glucosuria; this indicates a low renal threshold of glucose excretion. However, most patients with a positive reading have diabetes mellitus.
The dip-strip test for hemoglobin is not specific for erythrocytes and should be used only to screen for hematuria, with microscopic analysis of the urinary sediment used for confirmation. Free hemoglobin or myoglobin in the urine may give a positive reading; ascorbic acid in the urine can inhibit the dip-strip reaction and give a false-negative result. Note that dilute urine (<1.008) will lyse erythrocytes and thus provide a positive dip-strip reading for hemoglobin but no visible erythrocytes on microscopic analysis.
Test strips to determine the number of bacteria (nitrite) or leukocytes (leukocyte esterase) as predictors of bacteriuria are as accurate as microscopic sediment analysis in studies using quantitative urine cultures as the standard. The nitrite reductase test depends on the conversion of nitrate to nitrite. Many of the bacteria responsible for urinary tract infections, particularly enterobacteria, are capable of reducing nitrate to nitrite and, therefore, detectable by this test. When the nitrite test is positive, it suggests the presence of >100,000 organisms per milliliter; however, several factors can lead to false-negative results. The nitrite test is positive only for coagulase-splitting bacteria, and thus, when used alone it is only 40–60% accurate. Urine must be in the bladder for a sufficient time before sampling for the reduction of nitrate to occur (>4 hours); therefore, this test is most likely to be positive when first-voided morning urine is tested. A false-negative test will also result if the bacteria present do not contain nitrate reductase or if dietary nitrate is absent. A false-negative nitrite study may occur in a patient taking vitamin C. The leukocyte esterase test is a widely used chemical test that depends on the presence of esterase in granulocytic leukocytes. The leukocyte esterase test is an indication of pyuria and will remain positive even after the leukocytes have degenerated. The test accurately identifies patients with 10–12 leukocytes per high-power field in a centrifuged specimen. Although this test is a good indicator of pyuria, it does not detect bacteriuria. Therefore, it is often combined with the nitrite test to detect both bacteriuria and inflammation to maximize the chances of predicting urinary tract infection. Used together, the two tests are as predictive as the microscopic analysis but not as accurate as a urine culture. A false-negative leukocyte esterase study can be caused by glucosuria, or by phenazopyridine hydrochloride (Pyridium), nitrofurantoin, vitamin C, or rifampin in the urine.
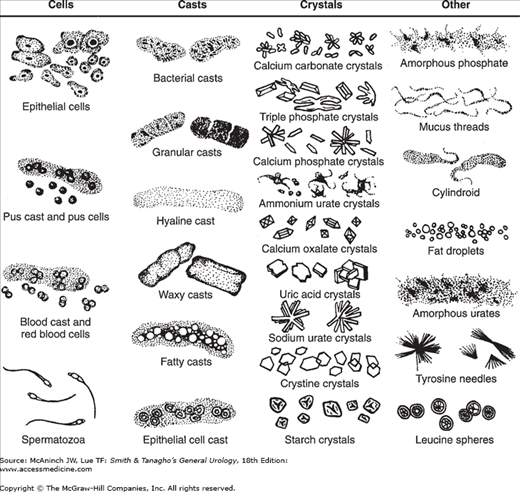
To be most accurate, the microscopic sediment examination should be done personally by an experienced physician or technician. Early-morning urine is the best specimen if it can be examined within a few minutes of collection. In most cases, the sediment can be prepared as follows: (1) centrifuge a 10-mL specimen at 2000 rpm for 5 minutes; (2) decant the supernatant; (3) resuspend the sediment in the remaining 1 mL of urine by tapping the tube gently against a countertop; and (4) place one drop of the mixture on a microscope slide, cover with a coverslip, and examine first under a low-power (10×) lens and then under a high-power (40×) lens. For maximal contrast of the elements in the sediment, the microscope diaphragm should be nearly closed to prevent overillumination. Significant elements (particularly bacteria) are more easily seen if the slide is stained with methylene blue, but staining is not essential. Figure 5–1 shows typical findings in the urinary sediment.
The significance of bacteria in the urinary sediment is discussed in Section “Bacteriuria.”
Just as the presence of bacteria in the sediment is not an absolute indication of infection, neither is the finding of pyuria. In the sediment from clean-voided midstream specimens from men and those obtained by suprapubic aspiration or catheterization in women, a finding of more than five leukocytes per high-power field is generally considered abnormal (pyuria). If the patient has symptoms of a urinary tract infection as well as pyuria and bacteriuria, one is justified in making a diagnosis of infection and initiating empiric therapy. However, in female patients with symptoms of urinary tract infection, 60% of those with pyuria will have no bacterial growth from bladder urine obtained by catheterization or suprapubic aspiration emphasizing the need for confirmation by bacterial cultures.
Renal tuberculosis can cause “sterile” acid pyuria and should be considered in any patient with persistent pyuria and negative results on routine bacterial cultures. Specific fluorescent staining of the urinary sediment for acid-fast bacteria can be diagnostic; however, results will be positive from the sediment of spot specimens in only approximately 50% of patients with renal tuberculosis, whereas they are positive in the sediment of 24-hour specimens in 70–80% of such cases. Mycobacterium smegmatis, a commensal organism, may be present in the urine (particularly in uncircumcised men) and can give false-positive results on acid-fast stains.
Urolithiasis can also cause pyuria. In patients with persistent pyuria, the physician should consider obtaining at least a plain x-ray of the abdomen and possibly a CT urogram to determine whether urolithiasis is present. Similarly, a retained foreign body such as a self-induced bladder object or a forgotten internal ureteral stent can cause pyuria. A plain x-ray (KUB film) of the abdomen should reveal the offender.
The presence of even a few erythrocytes in the urine (hematuria) is abnormal and requires further investigation. Although gross hematuria is more alarming to the patient, microscopic hematuria is no less significant. Infrequent causes of hematuria include strenuous exercise (long-distance running), vaginal bleeding, and inflammation of organs near or directly adjoining the urinary tract, for example, diverticulitis or appendicitis. Hematuria associated with cystitis or urethritis generally clears after treatment. Persistent hematuria in an otherwise asymptomatic patient of either sex and any age signifies disease and is an indication for further testing. Studies indicated that approximately 20% of patients with hematuria will eventually be diagnosed with bladder cancer (Messing and Vaillancourt, 1990).
In patients with microscopic hematuria, a three-container method for collection of urine can provide information on the site of origin of erythrocytes. (1) Give the patient three containers, labeled 1, 2, and 3 (or initial, mid, and final). (2) Instruct the patient to urinate and to collect the initial portion of the urine stream (10–15 mL) in the first container, the middle portion (30–40 mL) in the second, and the final portion (5–10 mL) in the third. (3) Using methods described previously, centrifuge the three specimens individually, prepare slides of the urinary sediment (with or without staining), and examine the slides microscopically. If erythrocytes predominate in the initial portion of the specimen, they are usually from the anterior urethra; those in the final portion are generally from the bladder neck or posterior urethra; and the presence of equal numbers of erythrocytes in all three containers usually indicates a source above the bladder neck (bladder, ureters, or kidneys). It is important to collect the urine before physical examination (particularly before rectal examination in men) to avoid misleading results.
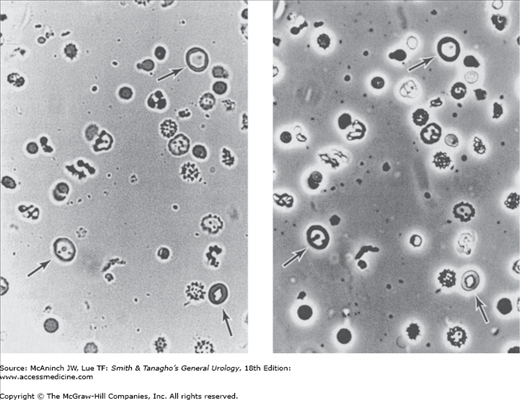
The three-container test may not be necessary in patients with gross hematuria, since the patients (men in particular) can usually tell the physician which portion of the stream contains the darkest urine (ie, the most erythrocytes). A specific dysmorphic erythrocyte configuration that can be detected with phase-contrast microscopy or by particle analyzer study of the urinary sediment and is highly indicative of active glomerular disease (Figure 5–2) can be useful. This dysmorphism is thought to be a result of extreme changes in osmolality and the high concentration of urinary chemical constituents affecting erythrocytes during passage through the kidney tubules. An automated system, iQ200, has been shown to be highly accurate for detecting, enumerating, and sizing erythrocytes in urine (Wah et al, 2005).
Figure 5–2.
Left: Dysmorphic erythrocytes in urine (arrows), viewed under light microscopy (magnification ×400). Right: Dysmorphic erythrocytes in urine (identical field), viewed under phase-contrast microscopy. (Reproduced, with permission, from Stamey TA, Kindrachuk RW: Urinary Sediment and Urinalysis: A Practical Guide for the Health Science Professional. WB Saunders, Philadelphia, PA, 1985.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree