Craig A. Peters, MD, FACS, FAAP
Pretransplant Assessment
Screening
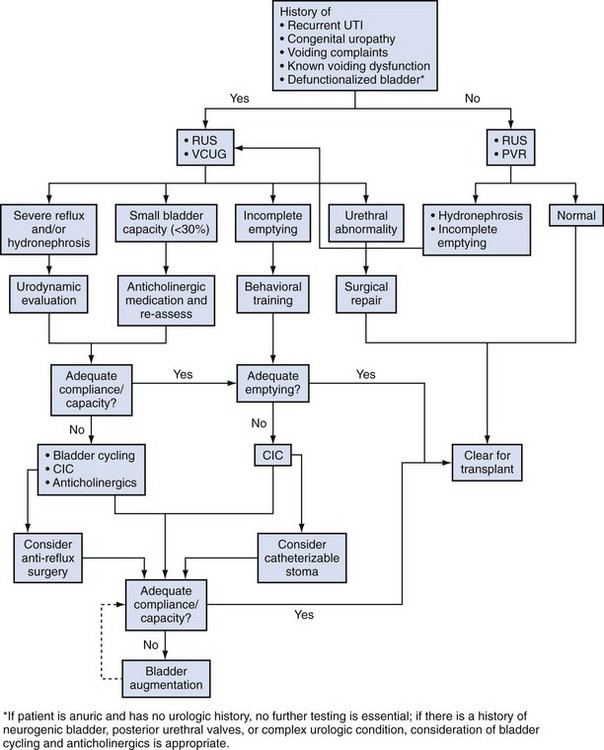
A large fraction of children in need of renal replacement will have some type of uropathy—either congenital obstruction, vesicoureteral reflux, or neuropathic bladder dysfunction (North American Pediatric Renal Transplant Cooperative Study [NAPRTCS], 2008). Younger boys will typically have obstructive uropathy, including posterior urethral valves, while the reflux and neuropathic bladder patients will be older, including young adults. Some children will have uncharacterized ESRD, and it is reasonable to have all children screened for urologic issues prior to transplant. A detailed history, renal ultrasonogram, and postvoid residual urine determined by ultrasonography (US) can effectively rule out most significant uropathies. A routine voiding cystourethrogram (VCUG) is not necessary, unless there is a history of a specific urologic disease, febrile or recurrent urinary tract infection (UTI), the presence of hydronephrosis, or clinically abnormal voiding (Ramirez et al, 2001) (Fig. 136–1).
Focused Assessment
The child with a known urologic abnormality will require a pretransplant assessment directed by the underlying condition and the status of the bladder and kidneys. In most, a VCUG will be useful to permit an assessment of voiding function, the state of the urethra, and the presence of reflux. When bladder dynamics are abnormal based on the VCUG or a renal US, consideration for urodynamic evaluation is appropriate. The indications for urodynamic testing to assess bladder capacity, compliance, and emptying, as well as sphincter function, includes a known neuropathic bladder abnormality, prior severe posterior urethral valves and any child with ongoing voiding dysfunction, hydronephrosis, or recurrent UTI (Burns et al, 1992; Zermann et al, 2003). The principle goal of the urodynamic testing is to determine the need for further therapy for bladder function. This might include medical therapy with anticholinergics, the use of intermittent catheterization, and the potential need for bladder augmentation in severe cases.
Key Points
Urologic Evaluation and Management
Pretransplant Preparation
Bladder Preparation
General Issues
The most common bladder abnormality associated with ESRD is the low-capacity, hypertonic bladder with poor compliance. This is the typical picture with posterior urethral valves (PUV), but it is important to recognize that these bladders continue to evolve and may progress to a pattern of insufficient contractility to empty at all (Peters et al, 1990; Nguyen and Peters, 1999). They may still be hypertonic and pose ongoing renal risk and will require CIC in most cases. Hypertonicity is the most dangerous dynamic pattern, as it will create an obstructive condition for the kidneys, even in the absence of reflux. Bladder dysfunction can increase graft loss (Herthelius and Oborn, 2007).
Hypertonicity
The hypertonic bladder is managed with medication as a first-line therapy, typically with anticholinergics, and with augmentation as the second-line therapy (Lopez Pereira et al, 2000). Medical management requires diligence on the part of the family and follow-up that regularly assesses the response to therapy. It will usually require a combination of anticholinergics and CIC, although some children with PUV can learn to void by the Valsalva maneuver. It should never be assumed that they will be able to do so; they must demonstrate this by repeated low postvoid residual volumes on catheterization.
Volume
Bladder capacity is another element of normal function and is the basis for both safe storage as well as social continence. As above, capacity can be improved with anticholinergic medication but may ultimately require bladder augmentation. There is a clear shift away from augmentation in all patients, and there have been prior reports that augmentation is not needed in renal transplant (Alfrey et al, 1997b; Salvatierra et al, 1999). However, there remains a role for this approach in children with complex uropathies with ESRD. Current indications would include nonsalvageable bladder (exstrophy, tumor, severe end-stage neuropathic bladder) and failure of medical and CIC therapy to achieve low-pressure storage for up to 3 hours. If pressures are safe for 1 hour only and they exceed 40 cm H2O the rest of the time, despite aggressive medical and catheterization therapy, consideration for augmentation is important.
There is no evidence or logic that bladder augmentation, itself, increases the risk of transplant failure, despite some reports (Alfrey et al, 1997b), and, indeed, it has permitted many effective transplants into very abnormal bladders (Sheldon et al, 1994; Taghizadeh et al, 2007). There is no single approach that is ideal, although gastric augmentation gained popularity for ESRD children in the late 1980s and early 1990s. It offers the benefit of secreting acid in a patient who is typically acidotic, and it can limit infection and has a lower rate of bladder stones. Gastric augments have, however, been linked with severe complications, particularly in the pre-transplant anuric group, due to gastric juice injury of the native bladder segment and due to the fairly common hematuria-dysuria syndrome (Reinberg et al, 1992; Nguyen et al, 1993). Recent reports of malignancy, particularly in the transplant group, are worrisome but are too anecdotal yet to change practices (Castellan et al, 2007; Husmann and Rathbun, 2008). Caution and monitoring are essential. Composite gastric augmenting patches are useful and may have less morbidity. Ileum and sigmoid are all useful and may be more appropriate in some patients, depending upon anatomy, prior surgery, and preferences for continent catheterizable stomas.
Infections
Many children with complex reconstruction of the urinary tract will have a history of recurrent UTI. This may be associated with intermittent catheterization. This is a potential hazard for the transplant, and pyelonephritis of the graft is certainly associated with graft loss (Dunn et al, 1987; Hanevold et al, 1987; Neuhaus et al, 1997; Howie et al, 2002; Herthelius and Oborn, 2007). The underlying etiology of the infection is most likely due to inadequate emptying of the reservoir, either by catheterization or voiding. This needs to be assessed and addressed in advance of the renal transplant. Strategies include double and triple voiding to avoid using CIC, or, if CIC has already been instituted, to ensure adequate emptying or to teach proper methods to ensure emptying.
Defunctionalized Bladder
Neuropathic Bladder
The defunctionalized neurogenic bladder is becoming scarce due to better management but will occasionally present clinically in a transplant patient who has not had ongoing urologic care (Firlit, 1976; Serrano et al, 1996). It is impossible to know without testing what the potential function of the bladder may be. It is also important to recognize that the bladder that has been defunctionalized will take some time to reach its maximal functional potential. This is often best accomplished by bladder cycling to increase capacity, determine bladder wall compliance, and assess the family’s ability to perform CIC.
Some have advocated simply implanting the transplant ureter into the bladder and anticipating normal function (Salvatierra et al, 1999). Although this may occur on rare occasions, it is a highly risky approach to the chronically defunctionalized bladder.
Cycling the defunctionalized bladder is best accomplished using a progressive program of catheterization with instillation of increasing volumes of saline, with a set dwell time, and then with catheter drainage (Alam and Sheldon, 2008). The amounts will be determined empirically based on initial tolerated volumes and should increase at regular intervals, usually 10 to 15 cc per day. The response to these instillations will give useful clues as to the utility of the bladder to serve as a reservoir and in its ability to empty spontaneously. Adjunctive anticholinergic medications are often necessary to increase bladder capacity and compliance.
The target volume is anticipated capacity for age based on any of the available formulas (Koff, 1983; Kaefer et al, 1997). This may not be reached immediately, but if volumes increase steadily without significant leakage, further expansion is likely. Although storage capacity is important, compliance is equally critical, and this must be assessed formally with urodynamics. The patient should be cleared for transplant only if capacity and compliance parameters near normal can be reached. Aggressive medical management is also important in this determination. If adequate storage and compliance cannot be attained, consideration for augmentation must be entertained. This should be performed prior to transplant, unless there are pressing contraindicating reasons.
The Decision to Augment
Although the use of bladder augmentation is declining due to concerns regarding metabolic, infectious, and neoplastic complications, enterocystoplasty remains the most effective means to provide normal bladder storage function in both neurogenic and postobstructive bladder dysfunction (Barnett et al, 1987; Sheldon et al, 1994; Hatch et al, 2001; Nahas et al, 2002; DeFoor et al, 2003; Capizzi et al, 2004; Mendizabal et al, 2005; Rigamonti et al, 2005; Aki et al, 2006). To lose a renal graft due to the same processes that contributed to native renal demise is unacceptable. The potential complications of augmentation must also be clearly recognized and anticipated, although some reports have presented extreme examples that are not the author’s experience (Alfrey et al, 1997a). A strategy of aggressive intermittent catheterization with medical management becomes the best approach to identify those patients in whom augmentation is the only real option for successful renal transplantation. It is difficult to determine a meaningful incidence of the need for augmentation, because the effort expended by both family and health-care team varies as much as the underlying pathology. With early aggressive bladder management, the need for augmentation in both neurogenic and obstructive bladder dysfunction has been declining. When bladder reconstruction using engineered tissues becomes more widely available, this will be a particularly valuable use.
The availability of a dilated ureter to permit ureterocystoplasty (Kim et al, 1996; Kurzrock et al, 2002) should be explored, although results have been mixed. It is preferable to perform enterocystoplasty in this context, and efforts should be made to preserve the ureter, when possible, for this use or for a continent catheterizable channel.