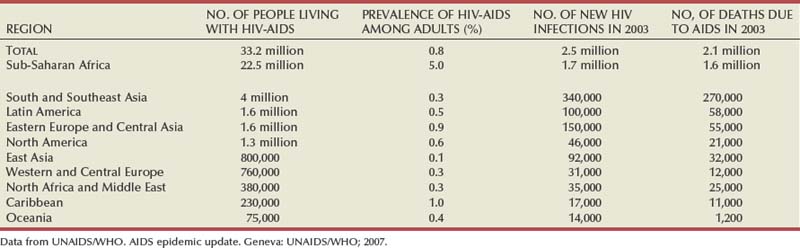
Thomas J. Walsh, MD, MS, John N. Krieger, MD After AIDS was described in 1981 the number of cases increased rapidly (Steinbrook, 2004; World Health Organization, 2004). An estimated 30.6 million to 36.1 million people worldwide are living with HIV infection. More than 20 million have died of AIDS (Joint United Nations Program on HIV/AIDS, 2004; UNAIDS/WHO, 2008). In 2007 alone, 2.5 million people were infected and 2.1 million died of AIDS. Of all people 15 to 49 years of age worldwide, 1.1% are now infected with HIV (Steinbrook, 2004). In less than 15 years HIV infection reached pandemic proportions, with AIDS reported in over 190 countries (UNAIDS/WHO, 2008). The impact of HIV infection is much different in the developing world than in the industrialized world. At one time AIDS was the leading cause of death among 25- to 44-year old men in Western European and North American cities and was the third most common cause of death among young women (Carael et al, 2004; Steinbrook, 2004). With effective antiretroviral therapy, deaths attributed to AIDS are declining rapidly. Worldwide, AIDS-related deaths declined from 2.9 million to 2.1 million from 2004 to 2007 (UNAIDS/WHO, 2008). The three main modes of HIV transmission have changed little: unprotected intercourse, contact with blood, and transmission from mother to child. Direct blood contact, such as sharing drug-injection equipment, results in the most efficient transmission. Globally, “unprotected” sexual intercourse between men and women is the predominant mode of HIV transmission (World Health Organization, 2004). The burden of HIV infection is greatest in the developing world (Steinbrook, 2004). Two thirds of HIV-infected persons are in Africa, where the epidemic exploded during the 1990s, and one fifth are in Asia, where the epidemic has been growing steadily (Steinbrook, 2004). Eight of nine countries with the most HIV-infected people are in sub-Saharan Africa. Estimates for India range from 2.2 to 7.6 million, and for China they are from 430,000 to 1.5 million. In comparison, an estimated 1.2 million people are living with HIV in the United States, 860,000 in the Russian Federation, and 680,000 in Brazil. Statistics highlight global disparities in availability of therapy. Overall, 1.6 million people died of AIDS in sub-Saharan Africa in 2007. By comparison, in Western and Central Europe, only 12,000 people died of AIDS in 2007 (Table 14–1) (Joint United Nations Program on HIV/AIDS, 2004; UNAIDS/WHO, 2007; Quinn, 2008). The prevalence of HIV has continued to rise in the United States with estimates as high as 1.2 million Americans living with HIV, nearly 75% of whom are adult men (Carael et al, 2004; Centers for Disease Control and Prevention, 2004; Steinbrook, 2004; Heyns et al, 2009). Early in the epidemic most infections occurred among men who have sex with men, but the incidence in this group leveled off by 1985-1987. However, HIV prevalence levels of 7% to 9% are still found among young homosexual and bisexual men in cities such as San Francisco and New York. The largest decline in the proportion of AIDS cases in the United States has occurred among homosexual and bisexual men, whereas cases acquired by heterosexual transmission have increased. Despite antiretroviral therapy, blood screening, and treatment of sexually transmitted infections the number of infections has remained at a plateau of 40,000 new HIV infections per year in the United States over the past decade (Mayer and Safren, 2004). The HIV prevalence among injecting drug users has been increasing steadily, but with large regional differences (Carael et al, 2004). Since the late 1980s on the U.S. west coast about 90% of people with AIDS are men who have sex with men, whereas on the northeastern coast most new HIV infections occurred among injecting drug users. Young adults belonging to ethnic minorities (including men who have sex with men) are at considerably greater risk of infection than they were 5 years ago. For example, African-Americans make up only 12% of the U.S. population but were affected in 47% of AIDS cases reported in 2000. HIV epidemics may occur suddenly, reflecting circumstances that are not fully understood (Carael et al, 2004). For example, HIV seroprevalence among injecting drug users in Bangkok increased from zero in 1985-1986 to 16% in 1988 and 40% to 60% in 1992. A number of the major risk factors are urologic (Table 14–2) (Carael et al, 2004). Early epidemiologic studies identified major risk factors, especially unprotected sexual intercourse with multiple partners or an infected partner, presence of sexually transmitted infections (STIs), or a history of STIs (Van de Perre et al, 1985; Kreiss et al, 1986; Cameron et al, 1989; Laga et al, 1994). More recent studies have highlighted sex differences in HIV transmission. For many monogamous women the main risk factor may be the sexual behavior of their steady partner (UNAIDS/WHO, 2008). Table 14–2 HIV Infection Risk Associated with Sexual Behaviors Compared with Blood Exposure Data from Henderson et al, 1986; Kaplan and Heimer, 1994; Royce et al, 1997; Varghese et al, 2002; and Henderson, 2004. Multiple cofactors affect HIV transmission, which is why estimates vary on the relative risk for specific exposures (see Table 14–2) (Royce et al, 1997; Vernazza and Eron, 1997). The probability of HIV transmission associated with unprotected vaginal sexual intercourse is not constant from one contact to another: estimates range from 0.0005 to 0.002 per episode, in the absence of cofactors (Carael et al, 2004). A recent meta-analysis of observational studies provided risk estimates for per sexual act transmission of HIV-1 among heterosexuals (Boily et al, 2009). The study included 43 publications, based on work in 25 different study populations. The pooled risk estimates suggest that female-to-male (0.04% per act [95% confidence interval [CI] 0.01-0.14]) and male-to-female (0.08% per act [95% CI 0.06-0.11]) HIV transmission rates are low in high-income countries. In contrast, much higher HIV transmission rates occur in low-income countries with female-to-male (0.38% per act [95% CI 0.13-1.10]) and male-to-female (0.30% per act [95% CI 0.14-0.63]) HIV transmission rates, even in the absence of commercial sex exposure. In multivariate analyses, transmission rates were influenced by gender, setting (high- vs. low-income countries), and antenatal HIV prevalence. As expected, the pooled risk estimate was higher for receptive anal intercourse (1.7% per act [95% CI 0.3-8.9]) and for the presence or history of genital ulcers in either couple member (5.3% per act [95% CI 1.4-19.5]). The presence of STIs suggests a marked risk of concurrent HIV. First, the modes of transmission of HIV and other STIs are similar. Second, genital ulcers as well as nonulcerative STIs facilitate HIV transmission (Cameron et al, 1989). Ulcerative STIs, including herpes, syphilis, and chancroid, enhance susceptibility to HIV per sexual act. Nonulcerative STIs, including gonorrhea, chlamydial infections, and trichomoniasis, are independent risk factors for HIV, with relative risks of 2.7 to 3.5 (Laga et al, 1994). Because syphilis facilitates acquisition and transmission of HIV, recent outbreaks of syphilis among men who have sex with men in major U.S. cities and reported increases in sexual behavior have raised concerns about potential increases in HIV transmission (Centers for Disease Control and Prevention, 2004). Several studies report conflicting results for aggressive diagnosis and treatment of STIs to limit HIV infection in high-risk populations. One study from Uganda employed syndromic management of STIs in an area where the epidemic was in its early stages with less than 1% of the adult population being infected at study inception. HIV incidence decreased in communities where the intervention was undertaken compared with control communities (Grosskurth et al, 1995). Different findings were reported in a study from Tanzania that employed periodic mass STI treatment of at-risk adults. This study found no decrease in HIV incidence (Wawer et al, 1999). In the later study the epidemic was more advanced with more than 16% of the adult population being infected at study inception. In addition there was a high rate of concomitant genital herpes that was not treated. Taken together, these findings suggest that efforts to decrease HIV spread through treating STIs should focus on specific treatment for individual patients, with aggressive diagnosis and treatment of STIs that are common in the community. The potential benefit of STI control programs may also prove greatest in areas of lower HIV prevalence. In communities where the epidemic is widespread, the likelihood of encountering a new partner who is HIV infected may be substantial, so the benefit of modifying a cofactor may be more limited (Mayer and Safren, 2004). Only a small proportion of sexual exposures result in HIV transmission (Anderson and May, 1988). Transmission may occur by exposure to cell-free or cell-associated virus, and different factors may affect expression of virus concentrations in different body fluids (i.e., blood, semen, or cervicovaginal secretions) (Krieger et al, 1998; Chakraborty et al, 2001; Mayer and Safren, 2004). Although lower blood HIV levels are associated with lower transmission rates (Quinn et al, 2000), antiretroviral therapy may not make HIV-infected people noninfectious. In fact, sexual transmission of multidrug-resistant HIV has been well documented (Boden et al, 1999; Little et al, 1999). These observations underscore the need for HIV-infected patients to practice safer sex even if they are on therapy. Three lines of evidence support the conclusion that circumcised men are at lower risk for HIV infection: biologic evidence, data from observational studies supported by meta-analyses, and the results of three randomized clinical trials supported by meta-analyses (Table 14–3) (Weiss et al, 2000). Table 14–3 Evidence Table for Studies of Male Circumcision and HIV Infection Risk That Include Original Data, Systematic Reviews or Meta-Analysis, Expert Opinion, or Other Human Data (1999-2008) The biologic mechanisms that could explain the increased risk of HIV among uncircumcised men include an increased rate of inflammatory conditions, susceptibility of the mucosal surface of the prepuce to trauma, and the longer survival of pathogens in the warm, moist subpreputial space. The inner foreskin is particularly susceptible to HIV infection owing to lack of keratinization and the high density of HIV target cells that are readily accessible (Patterson et al, 2002; Soilleux and Coleman, 2004; McCoombe and Short, 2006). The hypothesis that male circumcision might protect against HIV infection was first suggested in 1986 (Fink, 1986). Subsequent support was provided by ecologic studies in areas with low prevalence of male circumcision and high HIV prevalence in sub-Saharan Africa in the late 1980s (Moses et al, 1999) and later across 118 developing countries (Drain et al, 2006). Further evidence comes from two systematic reviews of the observational study data comparing HIV infection rates in circumcised and uncircumcised men from the same populations (Weiss et al, 2000; Siegfried, 2005). One review was restricted to 27 studies from sub-Saharan Africa (Weiss et al, 2000), and the other was a global review including 37 studies (Siegfried, 2005). Circumcised men had substantially lower risk of HIV infection. Meta-analysis of the 15 studies that adjusted for potential confounders showed this reduction to be large and highly significant (adjusted risk ratio of 0.42, 95% CI 0.34-0.54) (Weiss et al, 2000). Subsequent studies have found similar large risk reductions among circumcised men (Weiss et al, 2008). Although provocative, observational studies cannot prove causality. Three randomized clinical trials of adult male circumcision among consenting, healthy men in South Africa (Auvert et al, 2005), Kenya (Bailey et al, 2007), and Uganda (Gray et al, 2007) were started in 2002-2003. All three trials were stopped early after recommendations by independent data and safety monitoring boards when interim analyses found highly significant decreases in HIV infection risk among participants randomly assigned to circumcision. The three trials enrolled a total of 10,908 uncircumcised, HIV-negative adult men. Participants were randomly assigned to circumcision or control arms and then observed for up to 2 years. Retention rates were high (86% to 92%). Subsequent meta-analysis using a random-effects model of the trial results, following the QUORUM statement recommendations, found no evidence of heterogeneity among the trials (Weiss et al, 2008). The overall risk ratio was 0.42 (95% CI 0.31-0.57), corresponding to a protective effect of 58% (95% CI 43%-69%), which was identical to that found in the observational studies (58%, 95% CI 46%-66%). The true protection provided by male circumcision may be better estimated by an “as-treated” analysis, assigning outcomes according to the actual circumcision status of participants. All participants did not adhere to the study arm to which they were randomly assigned. Meta-analysis of the “as-treated” results of the three trials shows even stronger protection against HIV infection in the circumcision group (summary risk ratio of 0.35; 95% CI 0.24-0.54) (Weiss et al, 2008). Additional data have suggested that circumcision is both highly effective at reducing costs of HIV and AIDS and has a very low complication rate (Krieger et al, 2005, 2007; Gray et al, 2007). In summary, the positive findings in the male circumcision trials are in contrast to recent negative results with other HIV prevention interventions, including microbicides, the female diaphragm and gel, treatment to suppress genital herpes infections, and, most recently, an adenovirus-based HIV vaccine. There is a need to provide safe male circumcision services for high-risk populations because this is one of few proven HIV prevention strategies. In addition to other health benefits, male circumcision provides a much-needed addition to the limited HIV prevention armamentarium. Male circumcision is possibly the oldest and the most common surgical procedure. The evidence from biologic studies, observational studies, randomized controlled clinical trials, meta-analyses, and cost-effectiveness studies is conclusive; the challenges of implementation in high-risk populations must now be faced. Female genital tract tissues vary in susceptibility to HIV infection. The stratified vaginal epithelium contains fewer cells with coreceptors that can bind HIV (Patterson et al, 1998). Thus, vaginal mucosa is less susceptible to HIV infection than the endocervix, which has a thinner, highly vascular layer, containing a much higher concentration of HIV-binding cells. Physiologic events that result in ectropion (i.e., increased exposure of the endocervix), such as the use of hormonal contraceptives or occult Chlamydia trachomatis infection, increase susceptibility to HIV (Mostad et al, 2000; Moscicki et al, 2001). Calculation of the precise risk for infection for each type of exposure is imprecise because many cofactors alter the amount of HIV in the genital tract (Mayer and Safren, 2004). Factors such as a source with a high plasma viral load (Quinn et al, 2000) or concomitant STI can greatly increase the average per-contact risk (Cohen et al, 1997; Wawer et al, 1999). Data used to generate per-contact risks are often based on cohort studies in which individuals recollect their risk during previous time intervals (often every 3 to 6 months). Recollections are often obtained about behaviors under the influence of drugs or alcohol. Data from the developed world suggest that men are more likely to transmit HIV to their female partners (see Table 14–2) (Mayer and Safren, 2004). However, studies from Africa suggest that rates of male-to-female and female-to male transmission were similar (Quinn et al, 2000). Reasons for this apparent difference in the efficiency of female-to-male transmission may include different rates of male circumcision or the prevalence of other STIs. For anal intercourse the insertive partner is less likely to acquire HIV from an infected receptive partner than vice versa but there appear to be sufficient susceptible cells in the distal male urethra and foreskin such that an insertive partner is still at substantial risk. One study suggested that, on average, receptive anal intercourse was more than seven times as efficient at transmitting HIV as insertive anal intercourse (DeGruttola et al, 1989). Oral intercourse, either fellatio or cunnilingus, is a much less efficient way to acquire HIV. However, case reports show that oral exposure to ejaculate may transmit HIV (Mayer and Safren, 2004). The efficiency of oral transmission is less than that of unprotected vaginal intercourse, in the realm of less than 1 per 1000 contacts. However, animal studies demonstrate that HIV can be transmitted orally via lymphoid tissue in the oropharynx. Although HIV has been identified in pre-ejaculatory secretions there are no reliable case reports of HIV transmission through exposure to pre-ejaculate without exposure to semen. HIV has a spherical shape with an outer envelope, variable surface projections, and an icosahedral capsid containing ribonucleoprotein complexed with a core shell (Fig. 14–1) (Liang and Wainberg, 2004). The HIV projections consist of envelope glycoproteins loosely associated with a lipid bilayer. Beneath the lipid layer the matrix protein covers the internal surface of the viral coat. The capsid protein constitutes the shell of the cone-shaped core, and the nucleocapsid protein forms part of a nucleoid structure that also consists of reverse transcriptase, integrase, and two copies of the single-stranded viral genomic RNA (Liang and Wainberg, 2004). (Electron micrograph courtesy of the Centers for Disease Control and Prevention.) The HIV replication cycle is diagrammed in Figure 14–2 (Liang and Wainberg, 2004). (From Liang C, Wainberg MA. Virology of HIV. In: Cohen J, Powderly WG, Berkley SF, et al, editors. Infectious diseases. vol. 2, 2nd ed. Edinburgh: Mosby; 2004. p. 1251–5.) HIV initially attaches to the CD4 receptor on susceptible host cells. High-affinity interactions occur between the viral envelope glycoprotein and a specific region of the CD4 molecule (Pollard and Malim, 1998) on the surface of immature T cells and mature CD4+ T-helper cells. In addition to CD4, a variety of coreceptors and host immune cell membrane proteins may promote HIV entry into target cells after the initial binding (Cocchi et al, 1996; Liang and Wainberg, 2004). HIV exploits the host cell transcription and translation machineries to generate its own gene products. The primary RNA transcripts of the provirus are made by host cell RNA polymerase II. Cellular activation and proliferation signals result in the binding of transcription factors to the long terminal repeat and lead to increased rates of initiation of transcription. Tat and Rev are two key virion-encoded proteins that upregulate viral gene expression and replication, whereas HIV accessory proteins, including Nef, Vif, Vpu, and Vpr, are crucial determinants of HIV virulence (Emerman and Malim, 1998). Host cellular ribosomes translate proviral mRNA into viral proteins (Liang and Wainberg, 2004). The HIV glycosaminoglycan proteins are the driving force for virus assembly. Viral reverse transcriptase and protease enzymes have been the main targets of anti-HIV therapy. Viral reverse transcriptase has an exceedingly high error rate (i.e., about one mutation/virus replication event) (Liang and Wainberg, 2004); thus mutants are constantly generated that have the potential for drug resistance. Drug resistance to HIV occurs when these mutations result in altered forms of HIV reverse transcriptase and protease that function yet are not inhibited by antiviral agents. The course of HIV infection is highly variable. In the absence of treatment the typical course occurs over 8 to 12 years. Three distinct phases have been defined: primary infection, chronic asymptomatic infection, and overt AIDS (Pierre-Alexandre and Pantaleo, 2004). Diagnosis of primary HIV infection depends on diagnostic testing. There are three laboratory characteristics: high plasma viremia often greater than 1 million HIV RNA copies/mL, a decrease in the blood CD4+ T-cell count, and large increase in the blood CD8+ T-cell count. Subsequently, a marked decline in plasma viremia coincides with resolution of the clinical syndrome (Kahn and Walker, 1998). This decrease in viral load also correlates with the appearance of the virus-specific immune responses, particularly HIV-specific cytotoxic T cells, indicating that immune responses downregulate virus replication (Pantaleo et al, 1994; Musey et al, 1997). Standard laboratory assays used to diagnose HIV infection are usually negative during acute infection. This apparent clinical “stability” is deceptive. Although viral levels and the blood CD4 T-cell count are stable, this stability is only apparent in the blood. Virus replication and accumulation of extracellular virus trapped in the follicular dendritic cell network continue actively in the lymphoid tissue, where progressive anatomic and functional deterioration occurs, leading to impaired specific immune responses (Pantaleo et al, 1993, 1998). Eventually, immunologic deterioration is reflected by a rapid increase in viremia levels and a drop in CD4+ T-cell counts, resulting in transition to overt AIDS. The clinical course of HIV infection is variable (Pierre-Alexandre and Pantaleo, 2004). Conceptually, patients may be classified in four categories: typical progressors, rapid progressors, slow progressors, or nonprogressors. In 60% to 70% of HIV-infected patients the median time between infection and the development of AIDS is 10 to 11 years, in the absence of therapy. These infected persons are “typical progressors.” Their clinical course is described earlier. The most common route of HIV infection is sexual transmission at the genital mucosa (Royce et al, 1997). Animal models provide important insights into the early pathogenic events. After intravaginal infection of rhesus monkeys, tissue dendritic cells (i.e., Langerhans cells that reside in the lamina propria adjacent to the vaginal epithelium) are the first potential target cells of HIV (Spira et al, 1996). These dendritic cells constitute a complex and highly developed system of antigen-presenting cells that are able to prime naive T cells. The ability of dendritic cells to attract and prime naive T cells can be explained by expression of a type II membrane protein with an external mannose binding, C-type lectin domain, named DC-Sign (Steinman, 2000). It has been suggested that interaction between DC-Sign and the intracellular adhesion molecule-3 represents the initial contact between dendritic cells and resting T cells, a critical step for initiation of T-cell immune responses. Dendritic cells express high levels of specific chemokines that target naive rather than memory T cells (Cameron et al, 1996). After encountering HIV below the vaginal epithelium, Langerhans cells either can be infected or can pick up HIV virions (Steinman, 2000). Thus DC-Sign–positive dendritic cells play the major role in the delivery of HIV to T cells, greatly amplifying the viral infection (Zhu et al, 1996). Subsequently dendritic cells migrate to the internal iliac lymph nodes where they target the T-cell areas and then present the viral antigens to activated virus-specific T cells. Therefore, dendritic cells play a key role, both in priming the initial HIV-specific immune response and in transporting HIV to the draining lymphoid tissue. Rapid recruitment and spreading of target cells (i.e., activated CD4+ T cells) confers a major advantage to HIV because these events occur before the appearance of virus-specific immune responses. Within 2 days after infection, HIV can be detected in the draining lymphoid tissue. The virus then disseminates rapidly throughout the lymphoid system. Afterward, HIV enters the bloodstream, where viral replication can be detected in plasma 5 days after infection in the animal model. In humans the time from mucosal infection and initial plasma viremia varies, ranging from 4 to 11 days (Pierre-Alexandre and Pantaleo, 2004). The clinical importance of these observations is that the risk of infection is increased by conditions that decrease genital mucosal barriers, especially lesions caused by inflammatory or infectious diseases, such as cervicitis, urethritis, and genital ulcers. Events in the lymphoid tissue play a central role in HIV infection. During primary infection the peak number of virus-expressing cells in the lymph node occurs at the same time as the peak of plasma viremia or shortly precedes it (Brodie et al, 1999; Musey et al, 1999; Pierre-Alexandre and Pantaleo, 2004). With the transition from primary to chronic infection there is a switch from individual virus-expressing cells to virus trapped by the follicular dendritic cell network of lymph node germinal centers. This trapped virus becomes the dominant form of virus in lymph nodes, and this event is associated with a dramatic decrease in the number of individual cells expressing viral RNA, reflecting the role of the host immune system in partially containing HIV infection (Pantaleo et al, 1998). Early in primary HIV infection, vigorous virus-specific immune responses may contribute to both control of the initial peak of virus replication and reduction in plasma viremia (Musey et al, 1997; Kahn and Walker, 1998; Pantaleo et al, 1993, 1998). However, HIV-specific immune responses cannot control HIV or block the eventual progression to symptomatic disease. HIV differs from other viruses by targeting a broad spectrum of effector components of the antiviral immune response very early after infection (during the primary phase) and by rendering these mechanisms ineffective or reshaping them (Soudeyns and Pantaleo, 1999). HIV escapes the host immune response by both virologic and immunologic mechanisms. Virologic mechanisms include formation of a stable pool of latently infected CD4+ T cells containing HIV that is capable of replicating (Finzi et al, 1997; Wong et al, 1997), the genetic variability of HIV (Coffin, 1995), and trapping of infectious virions on the surface of follicular dendritic cells (Pantaleo et al, 1994; Chun et al, 1997; Wong et al, 1997). Initiation of HAART very early during primary HIV infection does have a significant impact on the size of this pool of CD4+ T cells. Decay of this pool of infected CD4+ T cells is very slow and not much influenced by the effective suppression of virus replication. This reservoir of replication-competent virus represents a major obstacle to HIV eradication and long-term control of virus replication (Chun et al, 1997; Finzi et al, 1997; Wong et al, 1997). HIV also reshapes antiviral mechanisms by trapping of the virus on the surface of follicular dendritic cells in lymphoid germinal centers. For most infections, formation of immune complexes and their attachment to the follicular dendritic cell network represent mechanisms leading to clearance of the pathogen and to maintenance of effective immune responses. However, with HIV infection these mechanisms lead to formation of a stable reservoir of infectious virions, representing a continuous source for infection of CD4+ T cells that ultimately results in the destruction of the lymphoid tissue (Musey et al, 1997). HIV escapes from the immune response through multiple immunologic mechanisms that include deletion of HIV-specific CD4+ T-cell clones (Rosenberg et al, 1997), deletion of HIV-specific cytotoxic CD8+ T-cell clones, generation of virus escape mutants (Soudeyns and Pantaleo, 1999), egress of cytotoxic T cells from lymph nodes, impaired function of antigen-presenting cells (Soudeyns and Pantaleo, 1999), and interference with the humoral neutralizing response. During the transition from primary to chronic infection, HIV plasma RNA levels reach a virologic set point that predicts the rate of disease progression (Mellors et al, 1996). The virologic set point varies among HIV-infected individuals and tends to remain stable in the same person during the chronic phase. The virologic set point that a person attains is determined by both the mechanisms involved in the establishment of chronic infection and by host factors that can modulate the course of HIV disease. The plasma RNA load is the most accurate predictor of disease progression (Mellors et al, 1996). The higher the plasma viral RNA load, the greater is the risk of rapid progression to AIDS and death. Free HIV virions are eliminated with a half-life of 6 hours. In contrast, productively infected cells have a half-life of 1.6 days. It was hoped that HIV eradication was achievable within 2 to 3 years (Perelson et al, 1997). This goal could only be realized with complete and sustained suppression of HIV replication and if there were no pool of long-lived, latently infected cells to serve as a virus reservoir (Chun et al, 1997; Finzi et al, 1997; Wong et al, 1997). Data from HIV-infected patients on long-term HAART significantly challenged these theories. There appears to be a pool of latent HIV–infected resting CD4+ T cells that persists in HIV-infected patients who adhered to HAART for up to 3 years (Chun et al, 1997; Finzi et al, 1997; Wong et al, 1997). This pool is composed of quiescent memory CD4+ T cells with a longer half-life than the original estimate of 1 to 4 weeks. More importantly, these cells contain replication-competent HIV proviral DNA. After activation these cells are able to support efficient viral replication. The reservoir of HIV-infected cells originates from productively infected cells during primary infection (Pierre-Alexandre and Pantaleo, 2004). Initiation of HAART as early as 10 days after the onset of symptoms of primary HIV infection rapidly controls plasma viremia but does not preclude generation of this viral reservoir. These observations emphasize the rapidity with which HIV viral reservoirs are established and the limited ability of HAART to interfere with this pathogenic process. This pool of HIV-infected cells represents a major obstacle to HIV eradication. In addition to their role as a viral reservoir, HIV-infected, long-lived, resting memory CD4+ T cells possess the ability to support virus production, albeit at low levels. Patients on HAART with viral loads below detectable levels with usual clinical assays may still have viral replication detected by more sensitive tests (Pierre-Alexandre and Pantaleo, 2004). In these studies, 40% to 60% of patients still had low levels of viremia after 36 to 48 weeks of HAART. Viral sanctuaries represent another potential source of residual HIV replication. Sanctuaries are defined as cells and organs where the HIV can be sheltered or where HAART does not achieve therapeutic concentrations. Tissue sanctuaries include the lymphoid tissue, mucosa-associated lymphoid tissue, the genital organs, and the central nervous system, where the achievable concentrations of antiviral drugs (especially protease inhibitors) may be suboptimal. These sites may serve both as potential sources of low-level virus replication and as reservoirs of latently infected cells (Pierre-Alexandre and Pantaleo, 2004). Presence of viral sanctuaries, including the reproductive tract, with limited bioavailability of antiviral drugs further complicates the issue of HIV eradication. Current estimates are that it may take 5 to 10 years or more to eliminate HIV, considering a half-life of 4 months for the long-lived infected CD4+ cells and provided that effective and durable suppression of viral replication is achieved by HAART. HIV was first isolated in 1983, and the first diagnostic tests were marketed in 1985. Tests for diagnosis and monitoring of HIV infection have improved constantly (Kuritzkes, 2004; Reiss et al, 2004). Few areas in medicine have witnessed such rapid and widespread adaptation of molecular tools to everyday practice. Fortunately the many tests for diagnosis and monitoring HIV infection can be considered in three categories: diagnostic tests, viral load, and resistance assays. Assays have been developed to detect HIV antibodies in serum, whole blood, saliva, and urine (Kuritzkes, 2004). Most laboratories screen for anti-HIV-1 and anti-HIV-2 antibodies using an enzyme-linked immunosorbent assay (ELISA) based on antigens from viral lysates, recombinant or synthetic. Current third-generation HIV ELISAs have sensitivity and specificity approaching 100%. In contrast to earlier tests that only detected IgG antibodies these tests detect all classes of anti-HIV antibodies, substantially shortening the time to diagnosis after acute infection. A variety of rapid tests and “home tests” have also been developed. Despite sensitivity of more than 99%, a reactive HIV ELISA has a relatively low positive predictive value in low-risk populations. Thus the current testing algorithm includes a confirmatory test to exclude false-positive results. The Western blot is most widely used for confirmation. Some laboratories prefer immunoblotting for confirmation because immunoblots are simpler to standardize and are more sensitive in cases of recent seroconversion (Kuritzkes, 2004). After acute infection, HIV RNA can be detected from day 12. HIV RNA assays (see later) have a sensitivity of 100% in diagnosing acute infection but at the cost of lowered specificity (97%) (Kuritzkes, 2004). The first antibodies can generally be detected on day 21. However, development of a positive HIV antibody test can vary according to the patient and infecting strain. Beyond week 6 after infection antibodies are detectable in almost all patients (Kuritzkes, 2004). Because reactivity by the Western blot test lags seroconversion by ELISA, a positive ELISA in a patient with a negative or evolving Western blot assay can provide evidence of recent infection. Assays to quantify HIV RNA, termed the viral load, led to our current understanding of HIV pathogenesis and helped establish complete suppression of HIV replication as the ultimate goal for antiretroviral therapy (Kuritzkes, 2004). These assays are now standard. Performance characteristics of the commercially available assays are similar. Current commercial assays have a lower limit of quantitation of 50 to 80 HIV RNA copies/mL. Plasma HIV RNA levels correlate with clinical stage (Kuritzkes, 2004). Patients with symptomatic HIV infection or AIDS have significantly higher virus loads than patients with asymptomatic infection. Plasma HIV RNA load is also a powerful predictor of the risk of disease progression and death at all stages of disease. Current guidelines recommend obtaining two plasma HIV RNA measurements to determine the baseline or “set point” virus load before initiating antiretroviral therapy. After antiretroviral therapy the change in plasma HIV RNA provides important clinical information. Several large clinical trials showed significant correlations between the plasma HIV RNA reduction from baseline and the clinical benefit (Marschner et al, 1998). The nadir in plasma HIV RNA levels is a marker for the duration of virus suppression (Raboud et al, 1999). The early response to treatment predicts long-term outcome. Conversely, increasing plasma HIV RNA level suggests treatment failure. Increasing plasma HIV RNA level is the harbinger of potential development of retroviral drug resistance (Reiss et al, 2004). This is not necessarily accompanied by an immediate decline in blood CD4+ lymphocyte numbers or by the development of clinical symptoms. Regular monitoring with plasma HIV RNA levels facilitates timely detection of drug-resistant HIV in patients on HAART. Various assays are available to assess drug resistance (Kuritzkes, 2004; Reiss et al, 2004). These assays can be considered as either genotypic or phenotypic assays. Genotypic assays evaluate HIV nucleotide sequences to detect critical drug-resistance mutations. Phenotypic assays estimate the concentration of antiviral drugs necessary to inhibit virus replication in vitro. Each approach has potential advantages and disadvantages. An important limitation is that both approaches measure characteristics of the predominant viral species but do not indicate the presence of minor species that may emerge as resistant variants during subsequent treatment. Clinical trials demonstrate the value of drug-resistance testing (Kuritzkes, 2004; Reiss et al, 2004). The risk of virologic failure during salvage therapy was reduced by 30% to 50% for each drug in the new regimen that was predicted to have activity (De Gruttola et al, 2001; Kuritzkes, 2004). Drug-resistance remained an independent predictor of the likelihood of treatment failure even after controlling for treatment history. Most experts recommend drug-resistance testing to guide the selection of salvage therapy for patients whose therapy is failing and in pregnant women (Hirsch et al, 2000). Resistance testing is also recommended for patients with acute HIV infection. Some experts also recommend that evidence for increasing transmission of drug-resistant HIV (Little et al, 2002) provides a rationale for testing of all patients before initiation of antiretroviral therapy where affordable (Kuritzkes, 2004). The genitalia may be involved in either early or late stages of HIV infection (Kho et al, 2004). In many populations the pattern of HIV acquisition parallels that of STIs (Quinn et al, 1988). Thus, testing for HIV is recommended in anyone with a diagnosed STI or at risk for STI (Centers for Disease Control and Prevention, 2002). Accurate diagnosis of STIs is of exceptional importance in individuals at high risk for HIV infection because presence of an STI increases the risk of both transmitting and acquiring HIV infection. These infections may have more atypical and prolonged clinical manifestations in people with HIV infection.
HIV/AIDS Epidemiology
Worldwide Perspective
Developed World Perspective
Dynamics of the HIV Epidemic: Importance of Urologic Risk Factors
ROUTE/TYPE OF EXPOSURE
RISK OF INFECTION MEAN/RANGE (%)
Transfusion of contaminated blood
84-100
Intravenous drug use (needle sharing)
0.8
Receptive anal intercourse
0.3-0.8
Insertive anal intercourse
0.04-0.1
Occupational needlestick exposure
0.28-0.33
Insertive vaginal intercourse
0.03-0.09
Receptive vaginal intercourse
0.005-0.02
Insertive oral intercourse
0.003-0.008
Receptive oral intercourse
0.006-0.02
Variable Rates of Sexual Transmission
Sexually Transmitted Infections
Antiretroviral Therapy and Genital Secretions
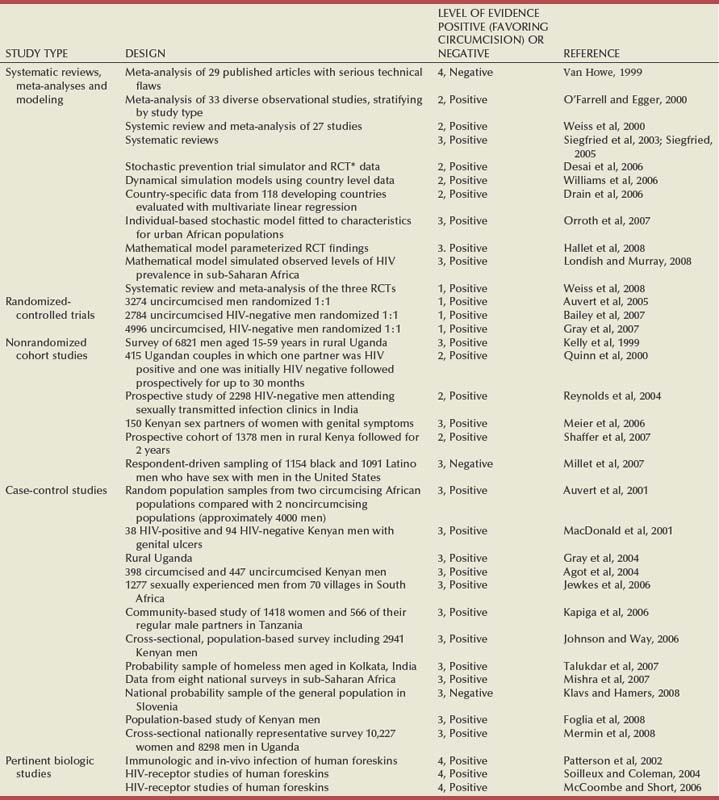
Circumcision Status
Gynecologic Factors
Specific Sexual Behaviors
HIV Virology and Targets for Antiviral Therapy
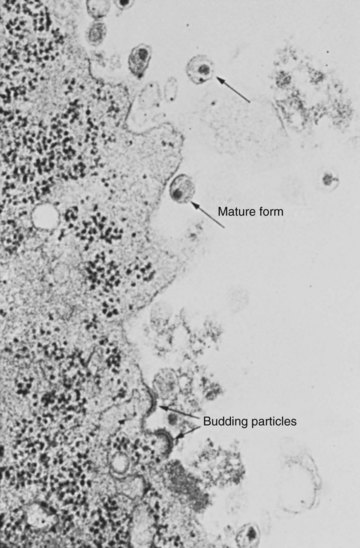
The Virion
HIV Replication Cycle
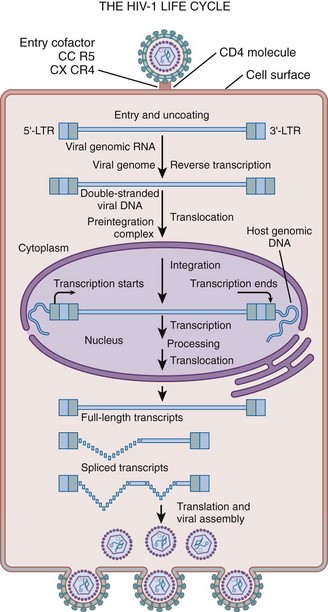
Viral Attachment, Fusion, and Uncoating
Viral Gene Expression, Packaging, and Assembly
Therapeutic Considerations
Pathogenesis of HIV Infection
Natural History of HIV Infection
Primary HIV Infection
Chronic Asymptomatic HIV Infection
Highly Variable Clinical Course
Early Pathogenic Events Determine the Natural History
Critical Role of Lymphoid Tissue in Primary HIV Infection
Virus Escape and Establishment of Chronic HIV Infection
Virologic Set Point
Prospects for HIV Eradication
Tests to Diagnose and Monitor HIV Infection: What the Urologist Should Know
Diagnostic Tests and Testing Algorithms
Clinical Application
Viral Load Monitoring
Clinical Application
HIV Drug-Resistance Assays
Clinical Application
Urologic Manifestations of HIV Infection
Nonmalignant Conditions
Sexually Transmitted Infections