Urodynamics
Victor W. Nitti
Melissa C. Fischer
INTRODUCTION
The lower urinary tract is responsible for the storage and evacuation of urine. Storage should occur at low pressure in order to ensure continence and protection of the kidneys, and evacuation should be voluntary. However, a variety of problems may arise that interfere with these two basic functions. Urodynamics (UDS) is the dynamic study of the transport, storage, and evacuation of urine by the urinary tract. It is comprised of a number of tests which individually or collectively can be used to gain invaluable information about lower urinary tract function.
The term “urodynamics” was first described by Davis in 1953, but the study of bladder pressure began in earnest in the late 19th century (1,2). Components of UDS include simple, noninvasive tests such as uroflowmetry to more sophisticated, invasive multichannel pressure-flow studies with sphincter electromyography and videofluoroscopy (videourodynamics). The following is a comprehensive review of UDS as it relates to the evaluation of the female patient. Furthermore, an emphasis will be placed on the diagnostic evaluation of urinary incontinence and pelvic floor prolapse. The terminology used conforms to the standards recommended by the International Continence Society (ICS), except where specifically noted (3).
INDICATIONS FOR URODYNAMICS
The initial evaluation of any patient includes a thorough history and physical and formulation of a differential diagnosis. While there have been many technological advances in the field of UDS, clinical expertise in deciding when, why, and how to perform the study is critical to the accurate interpretation and ultimate utility of the test. In general, UDS is indicated when the information and diagnosis provided will guide patient treatment. Examples of indications are an inconclusive diagnosis after simpler tests, a poor response to empiric therapy, presence of a condition with known deleterious effects (e.g., spinal cord injury or multiple sclerosis), or when a proposed treatment has significant risks. Women who have a combination of stress- and urge-related symptoms, who can poorly characterize their incontinence by history, who have undergone prior surgical procedures, or those in whom neurologic disease is suspected should strongly be considered for UDS prior to intervention. Studies have demonstrated that UDS may also be beneficial in women who report pure stress urinary incontinence prior to surgical intervention, as only 51% had pure urodynamic stress incontinence on urodynamic evaluation (4).
UDS is just one tool that can be used to assist in the diagnosis of genitourinary abnormalities and is best utilized when the clinician has specific questions to be answered. UDS is an interactive test between the clinician and the patient and should attempt to reproduce the patient’s symptoms. Often the objective data obtained is influenced by the circumstances and conditions of the test. Therefore, the ultimate interpretation of the data is subjective, requiring experience and an understanding of the patient’s history. Three general principles should always be remembered: (a) a study that does not reproduce the patient’s symptoms is nondiagnostic; (b) failure to record an abnormality does not rule out its existence; and (c) not all abnormalities are clinically significant (5).
A basic understanding of the physiology of urine storage and voiding and the pathophysiology of voiding dysfunction is required to formulate appropriate questions to be answered by an urodynamic study. However, all too often clinicians become caught up in the intricate neurophysiologic aspects of voiding and storage dysfunction and fail to think in practical terms. One should always focus on the possible urodynamic findings in a
given case and how each of the findings may ultimately affect treatment. The functional classification system described by Wein is a useful framework with which to conceptualize voiding dysfunction and characterize it based on urodynamic findings (6). Of equal importance is that treatment options can be guided by this system. The functional classification system is based on the simple concept that the lower urinary tract (comprising the bladder and the bladder outlet) must store and empty urine. For normal storage and emptying to occur, the bladder and bladder outlet must function in a proper and coordinated fashion. Hence, lower urinary tract dysfunction can be classified under the following rubrics: “failure to store,” “failure to empty,” or a combination of both. Urodynamic abnormalities may result from bladder dysfunction, bladder outlet dysfunction, or a combination of both.
given case and how each of the findings may ultimately affect treatment. The functional classification system described by Wein is a useful framework with which to conceptualize voiding dysfunction and characterize it based on urodynamic findings (6). Of equal importance is that treatment options can be guided by this system. The functional classification system is based on the simple concept that the lower urinary tract (comprising the bladder and the bladder outlet) must store and empty urine. For normal storage and emptying to occur, the bladder and bladder outlet must function in a proper and coordinated fashion. Hence, lower urinary tract dysfunction can be classified under the following rubrics: “failure to store,” “failure to empty,” or a combination of both. Urodynamic abnormalities may result from bladder dysfunction, bladder outlet dysfunction, or a combination of both.
URODYNAMIC TESTING
Uroflowmetry
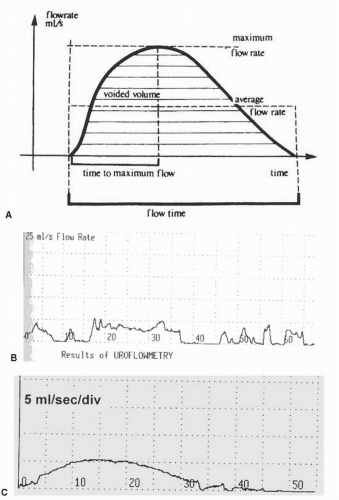
Uroflowmetry is a noninvasive means of quantifying the general effectiveness of voiding. The information may be used as an initial screening test or for comparison to monitor therapy but is not diagnostic as a single tool. Uroflowmetry is simple, noninvasive, and inexpensive. The test relies on the bladder being filled to normal capacity until the patient is comfortably full and has a normal desire to void. The patient is encouraged to sit and void as usual in a private setting. Prior to interpretation the patient should be asked whether the void was typical for her. We have found uroflowmetry particularly useful in women with significant voiding symptoms (decreased force of stream, hesitancy, straining to void) and incomplete bladder emptying.
The flow rate is directly related to the intravesical volume. The ideal volume for uroflowmetry is dependent upon the individual, but generally the volume should be greater than 150 mL for accurate interpretation (7). While we agree with the statement that a voided volume of 150 mL or more is optimal, we also realize that some patients cannot hold such a volume, and in these cases, the knowledge that the study was “typical” is important. Low-volume voids can be correlated with voiding diaries.
The urinary flow pattern is the result of the expulsion pressure, both detrusor and abdominal, and the outlet resistance. The following parameters can be measured during a noninvasive uroflow:
Flow rate: the volume of urine expelled via the urethra per unit time (mL/sec)
Voided volume: total volume expelled via the urethra (mL)
Maximum flow rate (Qmax): the maximum measured flow rate after correction for artifact (mL/sec)
Voiding time: the total duration of micturition, including interruptions (sec)
Flow time: the time over which measurable flow actually occurs (sec)
Average flow rate (Qave): voided volume divided by flow time (mL/sec)
Time to maximum flow: elapsed time from onset of flow to maximum flow (sec)
Postvoid residual volume (PVR) may be determined after uroflowmetry to assess how well the patient emptied her bladder. PVR may be measured by a bladder ultrasound or catheterization.
When interpreting an uroflow tracing, it is important to look at not only the objective parameters listed above but also the shape of the flow curve, which can give insight into the way the patient voids. The pattern of flow can be described as continuous or intermittent, smooth or fluctuating (3). A typical flow is a continuous, smooth, bell-shaped curve with high amplitude. A decreased detrusor contraction and/or increased outlet resistance will result in a lower flow rate and a smooth flat curve (8). Characteristic uroflow patterns are shown in Figure 6.1 (9,10).
How can uroflowmetry be applied to clinical practice? If a woman with significant voiding symptoms has a completely normal uroflow (rate and pattern) and a low PVR, then more invasive urodynamic testing may initially be deferred. Conversely, an abnormal uroflow might prompt further testing. An abnormal uroflow indicates that emptying is altered but is not diagnostic of etiology. Emptying abnormalities that affect uroflowmetry include impaired contractility and increased outlet resistance (obstruction) (11). A formal pressure-flow study is necessary to distinguish between the two. Uroflowmetry is particularly useful to evaluate a patient after an intervention that can affect emptying, such as anti-incontinence or prolapse surgery or urethrolysis for obstruction.
Cystometry
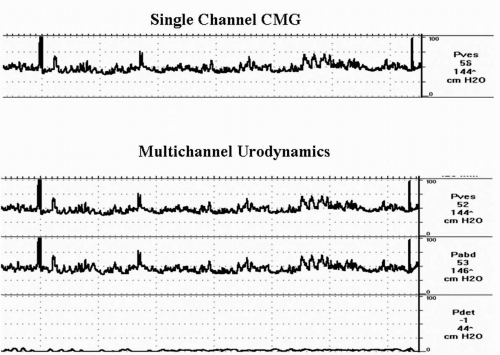
A cystometrogram (CMG) is a measure of the bladder’s response to being filled. It allows the clinician to determine the pressure-volume relationship within the bladder during bladder filling and storage of urine. A function of the bladder is to store increasing volumes of urine at low pressure. In addition, with the cooperation of the patient, it provides a subjective measure of bladder sensation. CMG provides correlation of patient’s symptoms with objective measures. Cystometry can be performed as a single-channel study where the bladder pressure (pves) is measured and recorded during filling and storage or as a multichannel study where abdominal pressure (pabd) is subtracted from pves to give the detrusor pressure (pdet). We believe that cystometry, whether done alone or as part of a pressure-flow study, is ideally done as a multichannel study with subtracted pabd (Fig. 6.2) (12).
It is beyond the scope of this chapter to describe all of the technical nuances of performing proper cystometry and UDS. The reader is referred elsewhere for more detail (13). However, it is important to remember several basic principles. First, the patient should be adequately prepared with an understanding of what to expect before the study is started. Intravesical catheters (usually 6 to 8 French) should be double- or triple-lumen to allow for both filling and simultaneous pressure measurement (bladder and urethra if desired). Abdominal pressure can be measured by placing a catheter in the rectum, vagina, or an abdominal stoma. Detrusor pressure (pdet) cannot be measured directly and therefore is a mathematically generated pressure (pves minus pabd) calculated automatically by the UDS computer software. The transducers should be at the level of the pubic symphysis, then zeroed to atmospheric pressure. If the transducers are not at the level of the pubic symphysis, then the baseline readings should be adjusted accordingly. At the beginning of the test, the patient is asked to cough to assess accurate transmission of pressure in both pabd and pves. If there is unequal transmission, then the catheters need to be adjusted or recalibrated prior to initiating the study. Typically medium fill is recommended
(10 to 100 mL/min), and we usually fill between 30 and 50 mL/min with normal saline or radiographic contrast for videourodynamics.
(10 to 100 mL/min), and we usually fill between 30 and 50 mL/min with normal saline or radiographic contrast for videourodynamics.
Several parameters may be evaluated during cystometry, including filling pressure, sensation, presence of involuntary or unstable contractions, compliance, capacity, and control over micturition.
Filling Pressure
Normally as the bladder fills it maintains a relatively constant and low pressure. Detrusor pressure usually does not exceed 5 to 10 cmH2O due to the vesicoelastic properties of the bladder; pdet remains low until the voluntary voiding phase. Rises in pdet may be caused by involuntary detrusor contractions (IDCs) or impaired compliance.
Sensation
Sensation is the part of cystometry that is truly subjective and therefore requires both an alert and attentive patient and clinician. Bladder sensation can be described in many ways. The ICS recommends judging bladder sensation by three defined points: first sensation of bladder filling, first desire to void (the feeling that would lead the patient to pass urine at the next convenient moment, but voiding can be delayed if necessary), and strong desire to void (persistent desire to void without the fear of leakage) (3). Patients can further be described as having normal, increased, reduced, or absent bladder sensation. Also, the ICS has provided terms to describe nonspecific bladder sensations, bladder pain, and urgency (a sudden compelling desire to void). If any of these sensations are experienced, the examiner should ask if they correlate with any of the patient’s symptoms.
Capacity
Cystometric capacity is the bladder volume at the end of the filling cystometrogram. The end point should be specified (e.g., the patient had a normal desire to void, a void was precipitated by detrusor overactivity, or the study was terminated for another reason). Maximum cystometric capacity is the volume at which a patient feels she can no longer delay micturition because of a strong desire to void (3). If during the study there is a question as to the bladder volume, the recorded instilled volume can be verified by adding the measured voided volume to the residual, which can be estimated by fluoroscopy or measured with ultrasound or a catheter.
Compliance
Bladder compliance is the change in bladder volume over a change in bladder pressure expressed in mL/cmH2O. Compliance is generally calculated by subtracting the baseline pdet from the premicturition pressure (pdet just prior to the initial isovolumetric contraction, also termed end-filling pressure) divided by the change in volume. Compliance is a reflection of the viscoelastic properties of the bladder, which normally allow storage of increasing volumes of urine at low pressures (see Fig. 6.2) (12). Abnormal or decreased compliance (increased pressure for a given volume) usually occurs in patients with underlying neurologic conditions, chronic catheterization, or certain inflammatory states. Decreased compliance is generally accepted to be less than 20 mL/cmH2O, which implies a poorly accommodating bladder (14). The absolute value of compliance is probably less important than premicturition pressure. Typically, pdet at the end of filling is 6 to 10 cmH2O (15). Clinically, it is most important to decide if the bladder is storing urine at elevated pressures for prolonged periods of time. An example of impaired compliance is shown in Figure 6.3.
Detrusor Contractions
The urodynamic observation of IDCs during the filling or storage phase is termed detrusor overactivity (DO) (Fig. 6.4) (16). DO may be phasic or terminal (occurring at maximum cystometric capacity). DO is usually, but not always, associated with an urge to void and may be associated with urgency or incontinence (DO incontinence). If an IDC is present, then the following should be noted: the volume at which the contraction occurred, the amplitude of the contraction, and if there was an associated leak. Furthermore, DO and DO incontinence are urodynamic observations, and the clinician must interpret the significance of these findings within the clinical context. Unequal transmission of pabd and pves or rectal contractions may falsely suggest an IDC. Careful attention to the tracings, patient activity, and associated rise in pabd should help delineate the situation.
According to the ICS, DO may also be described according to cause: neurogenic DO, when there is a relevant neurologic condition, and idiopathic DO, when there is no defined cause (3). The term “idiopathic” is a bit of a misnomer in that the cause of DO in a nonneurogenic patient may be readily apparent (e.g., bladder outlet obstruction or inflammatory process) versus truly “unknown.” Thus, from a practical standpoint, the terms “neurogenic DO” and “nonneurogenic DO” should be used.
Urodynamic stress incontinence is the involuntary leakage of urine during increased abdominal pressure in the absence of a detrusor contraction
(3). There are various urodynamic measurements of sphincteric function (see below), but the diagnosis of urodynamic stress incontinence per se can be made without any such measurements. During cystometry, filling can be stopped and the patient is asked to increase abdominal pressure by progressive Valsalva maneuvers or coughing. The demonstration of leakage with such maneuvers, in the absence of a detrusor contraction, confirms the diagnosis of urodynamic stress incontinence. Typically we start stress testing with cough or Valsalva at 150 mL of bladder volume and progress at 50-mL increments until stress incontinence is demonstrated or capacity is reached. The patient is asked to perform progressively forceful Valsalva maneuvers (Fig 6.5) followed by coughing (or other activity known to produce incontinence in a particular patient). Abdominal pressure at which leakage occurs is the abdominal leak point pressure (ALPP), but measurement of ALPP is not essential to make the diagnosis of urodynamic stress incontinence. In some cases it may be necessary to remove the urethral catheter in order to demonstrate stress incontinence.
(3). There are various urodynamic measurements of sphincteric function (see below), but the diagnosis of urodynamic stress incontinence per se can be made without any such measurements. During cystometry, filling can be stopped and the patient is asked to increase abdominal pressure by progressive Valsalva maneuvers or coughing. The demonstration of leakage with such maneuvers, in the absence of a detrusor contraction, confirms the diagnosis of urodynamic stress incontinence. Typically we start stress testing with cough or Valsalva at 150 mL of bladder volume and progress at 50-mL increments until stress incontinence is demonstrated or capacity is reached. The patient is asked to perform progressively forceful Valsalva maneuvers (Fig 6.5) followed by coughing (or other activity known to produce incontinence in a particular patient). Abdominal pressure at which leakage occurs is the abdominal leak point pressure (ALPP), but measurement of ALPP is not essential to make the diagnosis of urodynamic stress incontinence. In some cases it may be necessary to remove the urethral catheter in order to demonstrate stress incontinence.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree